Regents Chemistry - New York Science Teacher
... • Element X reacts with iron to form two different compounds with the formulas FeX and Fe2X3. To which group on the Periodic Table does ...
... • Element X reacts with iron to form two different compounds with the formulas FeX and Fe2X3. To which group on the Periodic Table does ...
Statics PPT
... How the Microfiber Works: Proper use of our microfiber cloths means that 99% of the bacteria are removed from surfaces. This is important in areas such as your bathroom and kitchen. The microfiber effectively removes dust, dirt, grease, chemical residues, and micro-organisms. The dry cloth employs s ...
... How the Microfiber Works: Proper use of our microfiber cloths means that 99% of the bacteria are removed from surfaces. This is important in areas such as your bathroom and kitchen. The microfiber effectively removes dust, dirt, grease, chemical residues, and micro-organisms. The dry cloth employs s ...
Can Quantum-Mechanical Description of Physical Reality be
... whole arrangement is therefore obviously unsuited to study the same kind of phenomena as in the previous case. In particular it may be shown that, if the momentum of the diaphragm is measured with an accuracy sufficient for allowing definite conclusions regarding the passage of the particle through ...
... whole arrangement is therefore obviously unsuited to study the same kind of phenomena as in the previous case. In particular it may be shown that, if the momentum of the diaphragm is measured with an accuracy sufficient for allowing definite conclusions regarding the passage of the particle through ...
Notes for Class Meeting 19: Uncertainty
... learned through relativity that space and time are closely related and form a fourdimensional spacetime. Therefore, if a particle’s position is uncertain, then a particle’s time must also be uncertain. Thus, it follows that time in quantum mechanics is fuzzy in the same way as space is fuzzy. Conseq ...
... learned through relativity that space and time are closely related and form a fourdimensional spacetime. Therefore, if a particle’s position is uncertain, then a particle’s time must also be uncertain. Thus, it follows that time in quantum mechanics is fuzzy in the same way as space is fuzzy. Conseq ...
Lecture 3 - Engineering
... UV-VIS absorption / fluorescence spectroscopy involves electronic energy transitions ...
... UV-VIS absorption / fluorescence spectroscopy involves electronic energy transitions ...
Accurate van der Waals interactions from groundstate
... (50 atoms and molecules – 1225 interaction pairs) ...
... (50 atoms and molecules – 1225 interaction pairs) ...
Module P11.4 Quantum physics of solids
... The nuclei of the atoms are electrically positive; their electrons are electrically negative. The force that holds the electrons to the atomic nuclei is electrical. The nuclei are dense concentrations of positive charge, confined within a region of space of radius of the order 10 −15 to 10−141m. The ...
... The nuclei of the atoms are electrically positive; their electrons are electrically negative. The force that holds the electrons to the atomic nuclei is electrical. The nuclei are dense concentrations of positive charge, confined within a region of space of radius of the order 10 −15 to 10−141m. The ...
Answers to NHSCE 2002 Part A Page 1
... amount is assumed to be evenly distributed throughout the solution. This is initially contained in a 250.00 mL of solution, but an aliquot of only 25.00 mL is taken, so that only 0.2500 mol x 25.00 mL/250.00 mL = 0.02500 mol are taken. The answer is therefore C. Incorrect answers involve calculation ...
... amount is assumed to be evenly distributed throughout the solution. This is initially contained in a 250.00 mL of solution, but an aliquot of only 25.00 mL is taken, so that only 0.2500 mol x 25.00 mL/250.00 mL = 0.02500 mol are taken. The answer is therefore C. Incorrect answers involve calculation ...
Worksheet 3A on Molecules
... Of the species listed, only O3 and CO are polar. CO is polar due to the difference in electronegativity between O and C; O3 is polar because it has 3 RHED and one lone pair on the central atom. This lone pair is an area where negative charge is concentrated, so this results in the molecule having an ...
... Of the species listed, only O3 and CO are polar. CO is polar due to the difference in electronegativity between O and C; O3 is polar because it has 3 RHED and one lone pair on the central atom. This lone pair is an area where negative charge is concentrated, so this results in the molecule having an ...
More Problems with Bohr
... This paper is a lead-in to an upcoming paper on the Rydberg series. I have recently thrown out all electron bonding theory and electron orbital theory, while creating a new model of the atom and a new diagram of the nucleus. Although my models are already quite convincing, they of course beg a large ...
... This paper is a lead-in to an upcoming paper on the Rydberg series. I have recently thrown out all electron bonding theory and electron orbital theory, while creating a new model of the atom and a new diagram of the nucleus. Although my models are already quite convincing, they of course beg a large ...
A Level Chemistry.pub
... Changes are under way for all A levels in all schools and colleges and some awarding bodies are still revising their syllabuses for 2015. As a result, this guide is an illustration of the content but the exact details may change. The most significant changes in A Levels and AS exams (but see below f ...
... Changes are under way for all A levels in all schools and colleges and some awarding bodies are still revising their syllabuses for 2015. As a result, this guide is an illustration of the content but the exact details may change. The most significant changes in A Levels and AS exams (but see below f ...
Calculation Booklet - Clydebank High School
... Enthalpy of Solution Enthalpy of solution of a substance is the energy change when one mole of that substance dissolves in excess water. Enthalpy of solution may be exothermic or endothermic. Worked Example (Note: the method is not always identical) 4g of ammonium nitrate, NH4NO3, is dissolved comp ...
... Enthalpy of Solution Enthalpy of solution of a substance is the energy change when one mole of that substance dissolves in excess water. Enthalpy of solution may be exothermic or endothermic. Worked Example (Note: the method is not always identical) 4g of ammonium nitrate, NH4NO3, is dissolved comp ...
CHEMISTRY 1710 - Practice Exam #2 (KATZ)
... water bath at 99°C. The barometric pressure is 753 torr. If the mass of the liquid retained in the flask is 1.362 g, what is its molar mass? a. ...
... water bath at 99°C. The barometric pressure is 753 torr. If the mass of the liquid retained in the flask is 1.362 g, what is its molar mass? a. ...
The return of pilot waves - Theory of Condensed Matter (Cambridge)
... distinguishing systems with identical Ψ (and possibly restoring determinism) are impossible. 3. Heisenberg’s uncertainty principle: observed fact that it is not possible to know values of all properties of system at same time; those properties not known with precision must be described by probabilit ...
... distinguishing systems with identical Ψ (and possibly restoring determinism) are impossible. 3. Heisenberg’s uncertainty principle: observed fact that it is not possible to know values of all properties of system at same time; those properties not known with precision must be described by probabilit ...
Chapter 3 Mass Relationships in Chemical Reactions
... 29. The molecular formula of aspirin is C9H8O4. How many aspirin molecules are present in one 500-milligram tablet? A. B. C. D. E. ...
... 29. The molecular formula of aspirin is C9H8O4. How many aspirin molecules are present in one 500-milligram tablet? A. B. C. D. E. ...
Print out Reviews # 1 through # 17
... 6. Sodium chloride can be prepared by the reaction of sodium metal with chlorine gas. (A) Write the balanced equation for the reaction: (B) 6.70 moles of Na reacts with 3.20 moles of Cl2 - What is the limiting reactant? - How many moles of NaCl are produced? EOC REVIEW # 10 1. What is the mass of ni ...
... 6. Sodium chloride can be prepared by the reaction of sodium metal with chlorine gas. (A) Write the balanced equation for the reaction: (B) 6.70 moles of Na reacts with 3.20 moles of Cl2 - What is the limiting reactant? - How many moles of NaCl are produced? EOC REVIEW # 10 1. What is the mass of ni ...
Observables - inst.eecs.berkeley.edu
... Since A corresponds to a conserved physical quantity, �ψ � | A |ψ � � = �ψ| A |ψ�. i.e. �ψ| U † AU |ψ� = �ψ| A |ψ�. Since this equation holds for every state |ψ�, it follows that U † AU = A. Substituting for U , we get LHS = e−iM t AeiM t ≈ (1 − iM t)A(1 + iM t) ≈ A − it[M, A] where [M, A] = M A − A ...
... Since A corresponds to a conserved physical quantity, �ψ � | A |ψ � � = �ψ| A |ψ�. i.e. �ψ| U † AU |ψ� = �ψ| A |ψ�. Since this equation holds for every state |ψ�, it follows that U † AU = A. Substituting for U , we get LHS = e−iM t AeiM t ≈ (1 − iM t)A(1 + iM t) ≈ A − it[M, A] where [M, A] = M A − A ...
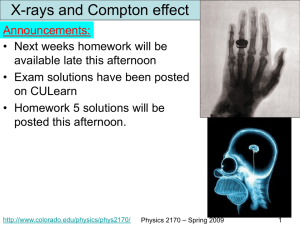
Physics 2170
... Compton effect We know that X-rays are just a part of the EM wave spectrum. In 1923 Compton published results showing that X-rays also behave like particles and that these photons have momentum. In classical theory, an EM wave striking a free electron should cause the electron to oscillate at the E ...
... Compton effect We know that X-rays are just a part of the EM wave spectrum. In 1923 Compton published results showing that X-rays also behave like particles and that these photons have momentum. In classical theory, an EM wave striking a free electron should cause the electron to oscillate at the E ...
Chemistry - NIC Karnataka
... Thermodynamic terms – concepts of system, surroundings, types of systems-examples, state of the system, state functions or state variables, energy- a state function, isothermal adiabatic, constant volume(isochoric)and pressure(isobaric) processes, reversible and irreversible processes, extensive and ...
... Thermodynamic terms – concepts of system, surroundings, types of systems-examples, state of the system, state functions or state variables, energy- a state function, isothermal adiabatic, constant volume(isochoric)and pressure(isobaric) processes, reversible and irreversible processes, extensive and ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.