* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Eddy Currents – Lesson Outline
Conservation of energy wikipedia , lookup
Time in physics wikipedia , lookup
Electrical resistance and conductance wikipedia , lookup
Condensed matter physics wikipedia , lookup
Nuclear physics wikipedia , lookup
Electromagnetism wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Superconductivity wikipedia , lookup
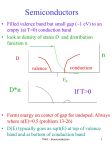
XXX ITDQIZTJDT FEVBV Eddy Currents – Lesson Outline Syllabus Reference 9.3.2.2.7 – explain the production of eddy currents in terms of Lenz’s Law. 9.3.2.3.4 – gather secondary information to identify how eddy currents have been utilised in electromagnetic braking. Resources Video: Eddy Currents http://www.hscphysics.edu.au/resource/EddyCurrents.flv Video: Magnet Race http://www.hscphysics.edu.au/resource/MagnetRace.flv Pre-video Activities Brainstorming activity: Students contribute key physics words pertaining to the production of an electric current by electromagnetic induction. Record words on board or screen. Matching exercise: Students should match key terms to definitions by matching the numbers 1-6 to the corresponding letters in the right-hand column. Key terms 1. Eddy currents Definitions A. An induced emf (voltage) always gives rise to a current whose magnetic field opposes the original change in flux 2. Lenz’s Law B. The amount of magnetic flux per unit area 3. Faraday’s Law C. A flow of charges induced in a conductor when there is relative movement between a magnetic field and a conductor 4. Induced current D. The production of a voltage across a conductor exposed to a changing magnetic field 5. Electromagnetic E. Circular currents which are induced in a conductor exposed to a induction changing magnetic field 6. Magnetic flux F. The induced emf (voltage) is equal to the rate of change of magnetic density !" flux interacting with a conductor Emf $ # n !t Group Work: Example: 1. Organise students into groups of three 2. Provide each group with a blank sheet of A3 paper and a marker 3. Students divide page into two with a horizontal line X X X X X X X X X X X X X X X X Have students draw a large square on the top half of the sheet. Describe the following situation: Imagine you are holding the north pole of a bar magnet 1 cm above the square. The square represents a square copper coil. Students draw lines of magnetic flux inside the square coil (a relatively strong magnetic flux density). "6 45 3 " -* " /NV MU J NFE JB GPSQIZTJDTTUVEFOUT 1 of 5 XXX ITDQIZTJDT FEVBV Have students draw another square of the same size on the lower half of the A3 page. Describe the new situation: the imaginary bar magnet is now lifted to a height of 5cm above the square coil. Students draw the magnetic flux lines inside the square for this new situation when the bar magnet is stationary 5 cm above the square. Students then write a statement to justify what they have drawn. Describe new situation: Imagine now how the flux lines change while the magnet is in motion from close to the square to a height of 5cm. The square copper coil contains free electrons in its lattice. Students amend their diagram to show what will happen to the electrons in the coil while the magnet is moving away. Students predict what will happen to the same electrons as the magnet is moved back down towards the square coil. Students show this on their diagram One representative presents the group’s interpretations to the rest of the class. Encourage class discussion and questioning Option 1: Pre-video Experiments For teacher background information, refer to video: Eddy Currents http://www.hscphysics.edu.au/resource/EddyCurrents.flv Students continue to work in groups of three. Give each group a rectangular sheet of galvanised steel and a rare earth magnet (with one half cut like a comb, as per video) Students firstly verify that the magnet is not magnetically attracted to the metal sheet. Students place the metal sheet so that it sits like a ramp and slide the magnet down the solid metal surface. Record observations. Students then slide the magnet down across the combs of the metal sheet. Record any observations and note any differences. For each of these investigations students should use a predict-observe-explain format. That is, they make a prediction before sliding the magnet, then record observations and generate plausible explanations. Format of tables: Situation 1: the magnet slides down the solid metal surface Prediction Observation Explanation Situation 2: the magnet slides across the metal combs Prediction Observation Explanation One representative presents the group’s interpretations to the rest of the class. Encourage class discussion and questioning. Collate all groups’ responses on board or screen. Back in groups, students draw models on A3 paper to illustrate how the eddy currents are induced in the metal sheet as the magnet slides "6 45 3 " -* " /NV MU J NFE JB GPSQIZTJDTTUVEFOUT 2 of 5 XXX ITDQIZTJDT FEVBV Again, one representative presents the group’s interpretations to the rest of the class. Ensure that different group members are presenting findings to class Option 2: View Video Video: Eddy Currents http://www.hscphysics.edu.au/resource/EddyCurrents.flv If unable to get the materials needed for option 1, students can watch the demonstrations in the video. View Video Video: Magnet Race http://www.hscphysics.edu.au/resource/MagnetRace.flv Activities Students again use the predict-observe-explain model to describe and interpret the results of the demonstration in the video. Each group should again report back to the class Students develop diagrams to model the production of eddy currents in the metal pipes Post-Video Activities Post-video Test: Eddy Currents - Extended Answer Students complete the post test individually to check for conceptual change. In groups of 3 students use the following key words to produce a concept map in groups. Lenz’s Law Eddy currents Induced current Electromagnetic induction Braking systems "6 45 3 " -* " /NV MU J NFE JB GPSQIZTJDTTUVEFOUT 3 of 5 XXX ITDQIZTJDT FEVBV Eddy Currents – Extended Answer Q1. Will an eddy current braking mechanism work on an aluminium wheel or should the wheel be made of a ferromagnetic material such as iron? Explain your answer. Q2. A thin, solid metal sheet is set up to hang from an axle so that it can swing freely (with minimal friction). The metal sheet is made to swing between the poles of a strong magnet. It soon comes to a stop after only one and a half swings. The metal sheet is then replaced by another one with the same properties, however this time the sheet has been cut so that it resembles a large comb. Predict what will happen when this new metal sheet is made to swing between the magnetic poles. Justify your prediction. Q3. An aluminium sheet is held between the poles of a large permanent magnet. It requires considerable force to pull it out from the magnetic field. Explain. "6 45 3 " -* " /NV MU J NFE JB GPSQIZTJDTTUVEFOUT 4 of 5 XXX ITDQIZTJDT FEVBV Q4. A magnet falling inside an aluminium pipe soon reaches a rather slow terminal velocity. Explain. Q5. After viewing this, a student asks his teacher if it is possible for the magnet to be brought to a complete stop so that it just sits suspended inside the vertical pipe. How would you respond to this student? Q6. Explain how eddy currents are applied in an induction stove top. "6 45 3 " -* " /NV MU J NFE JB GPSQIZTJDTTUVEFOUT 5 of 5 Eddy Currents 2 – Lesson Outline Syllabus Reference 9.3.2.2.7 – explain the production of eddy currents in terms of Lenz’s Law. 9.3.2.3.4 – gather secondary information to identify how eddy currents have been utilised in electromagnetic braking. Resources Video: Jumping Rings http://www.hscphysics.edu.au/resource/JumpingExplained.flv Video: Different Jumping Rings http://www.hscphysics.edu.au/resource/gzsod0ma0rjmczvi Video: Cold Jumping Rings http://www.hscphysics.edu.au/resource/sofrbwr4pkwr9ik7 Video: Double Jumping Rings http://www.hscphysics.edu.au/resource/rwf4n51t9svml0y2 Pre-video Activities Pre-video Activity: Jumping Rings Activity Sheet. Students are to complete the activity sheet individually. Because of the content of this topic, the Eddy Currents Lesson Outline would prove useful for the lead-up lesson. View Video Video: Jumping Rings http://www.hscphysics.edu.au/resource/JumpingExplained.flv Activities Split students into pairs. Hand out butcher’s paper and markers. Students draw a diagram of the set-up of the Jumping rings. In different colours, add the magnetic field lines, the conventional current in the electromagnet, the induced eddy current, and the induced magnetic pole. Students then label each diagram drawn with the physical law, i.e. the induced current is labelled with “Faraday’s Law” and the direction of the current is labelled “Lenz’s Law”. View Video Video: Different Jumping Rings http://www.hscphysics.edu.au/resource/gzsod0ma0rjmczvi 1 of 3 Activities Student draw diagrams of the four different rings. This time they also draw the difference in the eddy currents that are induced. The thicker rings should have a greater eddy current travelling through it- the rings with slits should have very small eddy currents. Lead a class discussion with the following questions: How does the material of the rings affect the creation of eddy currents? Are eddy current being produced in the slit-rings? Why is the induced magnetic field so small when slit-rings are used? View Video Video: Cold Jumping Rings http://www.hscphysics.edu.au/resource/sofrbwr4pkwr9ik7 Activities Students discuss in small groups the physical reasons why reducing the temperature of the metal decreases its resistance and why this leads the rings to jump higher. In groups, student’s answer the question: What happens to the rings if temperature is increased? View Video Video: Double Jumping Rings http://www.hscphysics.edu.au/resource/rwf4n51t9svml0y2 Activities Students work in groups with butcher’s paper and markers. Students can be given butchers paper to develop concept maps to explain what might be happening. Each group should present their work to the rest of the class. Encourage class discussion and questioning. Teacher collates all groups’ responses on board or screen. Ensure different group members are presenting findings to class. Post-video Activities As a summary or homework activity ask the students to write a hypothetical explanation of the jumping rings to a Year 11 student (keeping in mind the background knowledge such a student would possess). 2 of 3 Eddy Currents 2 Pre-Video Worksheet Fill in the blanks with the words provided below: electromotive change direction eddy opposes magnetic changing heat conductors Electromagnetic induction is a phenomenon in which circular fields ‘create’ . It is based on two principles; Faraday’s Law and Lenz’s electric currents in Law. magnetic fields induce an Faraday’s law states that force (which then creates a current in a conductor). Lenz’s law states the is a direction such that it that an induced current flows in a conductor – which the currents are a form of energy losses through that caused it. induced currents, which often cause generation. Match the change to its induced current and the induced magnetic field: CHANGE INDUCED CURRENT INDUCED MAGNETIC FIELD N S N S X X X X X X X X X X X X X X X X X X 3 of 3 XXX ITDQIZTJDT FEVBV Semiconductors – Lesson Outline Syllabus References 9.4.3.2.2 – describe the difference between conductors, insulators and semiconductors in terms of band structures and relative electrical resistance 9.4.3.2.6 - describe how ‘doping’ a semiconductor can change its electrical properties 9.4.3.2.7 - identify differences in p and n-type semiconductors in terms of the relative number of negative charge carriers and positive holes Resources Video: Effect of Temperature on Conductivity http://www.hscphysics.edu.au/resource/SemiHeat.flv Video: P-type and N-type Semiconductors http://www.hscphysics.edu.au/resource/t7v6ivgeg3pyjey3 Pre-video Activities Students contribute key physics words pertaining to the miniaturisation of electric circuits. Teacher records words on the board. Organise students into groups of three, hand out paper and a marker. Students list modern devices and appliances which have evolved from the miniaturisation of electrical components, such as integrated circuits and transistors. Students draw a timeline to show the evolution and development of technologies during the past century, from light bulbs to MP3 players. 1910 2010 Students list social and environmental impacts of these changes, both global and local. Representatives present the group’s responses to the rest of the class. Encourage class discussion and questioning. "6 45 3" -* " /NVM UJ NF EJ B G P S Q I Z T J D T T U V E F O U T 1 of 10 XXX ITDQIZTJDT FEVBV Students match key terms to definition by matching the numbers 1-6 to the corresponding letters in the right-hand column: Key terms 1. conductor 2. insulator 3. semiconductor 4. silicon 5. conduction band 6. electrical resistance 7. electrons 8. valence band 9. electric current Definitions A. A measure of the degree to which an object opposes an electric current passing through it. B. Fundamental subatomic particles that carry a negative electric charge. C. A material that readily allows the flow of an electric current D. In a solid lattice, this band is the highest range of energies where electrons can exist and still be held by their ‘local’ atom E. A material that resists the flow of electric current F. A metalloid from group IV of the periodic table. After oxygen, it is the second most abundant element in the Earth’s crust. It is the principal component of most semiconductor devices. G. A solid that has electrical conductivity in between that of a conductor and that of an insulator, H. The flow of electric charge per unit time through a material. I. The energy band higher than the valence band, where electrons are free to accelerate under the influence of an applied electric field View Video Video: Effect of Temperature on Conductivity http://www.hscphysics.edu.au/resource/SemiHeat.flv Activities Student Investigation during the video: Refer to video which demonstrates how the resistance changes when we heat a conductor, semiconductor and insulator. For each of these investigations students should use a predict-observe-explain format. That is, they make a prediction before viewing then record observations and generate plausible explanations. Format of tables: Situation 1: the metal (tungsten filament) is heated Prediction Observation Explanation Situation 2: the semiconductor (germanium rod) is heated Prediction Observation Explanation Situation 3: the insulator (rubber tubing) is heated Prediction Observation Explanation "6 45 3" -* " /NVM UJ NF EJ B G P S Q I Z T J D T T U V E F O U T 2 of 10 XXX ITDQIZTJDT FEVBV Back in groups, students graphically illustrate the relationship for resistance versus temperature for conductors and semiconductors R Temp Student groups write a conclusion for this investigation Again, one representative presents the group’s work to the rest of the class. Teacher ensures that different group members are presenting findings to class. Note: this experiment can easily be replicated in the school laboratory Advanced Activity Advanced Activity: Discussion of different explanations Advanced students read through the discussion paper in groups of three and discuss models to explain conductors, insulators, and semiconductors. View Video Video: P-type and N-type Semiconductors http://www.hscphysics.edu.au/resource/t7v6ivgeg3pyjey3 Activities Students make labelled diagrams to compare an n-type and p-type silicon lattice, modelling the behaviour of semiconductors. Students (in groups) create a model using bodies and chairs to demonstrate the movement of positive and negative charges in p-type and n-type semiconductors. Example: Line up 10 chairs and have the 9 people sit in a row leaving the chair on the right vacant; Explain that the external current terminals are positive to the right and negative to the left; The switch is turned on; The excited electrons (people) move (one chair at a time) through the crystal toward the positive terminal on the right; This shows the electrons from a bonded site move toward the positive terminal into an adjacent hole causing the hole to migrate toward the negative terminal (to the left). The hole can also be considered to be a charge carrier. Students organised into groups of three. Groups each create a concept map to show the connectedness of the following key terms: Integrated circuits Transistors Doping Extrinsic Intrinsic Solid state electronics Mobile phones Semiconductors Valence band Conduction band Energy gap Ipods Post-video Activities Activity: Semiconductors concept quiz and cloze passage. Students complete the activities individually. "6 45 3" -* " /NVM UJ NF EJ B G P S Q I Z T J D T T U V E F O U T 3 of 10 XXX ITDQIZTJDT FEVBV Semiconductors – Concepts Q1. The conduction of current within a p-type semiconductor is a result of: A. an excess electron B. a missing electron C. the substituted arsenic atoms D. the silicon atoms Q2. Why does the conductivity of silicon increase if it is doped with a group III element? A. The number of free electrons increases B. The number of positive holes increases C. The impurity decreases the energy band gap D. Additional electrons are added to the crystal lattice structure Q3. Which statement below best explains why metals have low resistance at room temperature? A. The conduction band has much greater energy than the valence band B. The valence band has much greater energy than the conduction band C. There are many electrons available for conduction D. There are few electrons available for conduction Q4. A semiconductor is doped with a small amount of boron. This means that: A. It is negatively charged, and so will conduct electricity more easily B. It is positively charged, and so will conduct electricity more easily C. It is neutral, but has more electrons available for conduction D. It is neutral, but has more holes available for conduction Q5. Which one of the following statements is NOT true in regards to a hole in semiconductor theory? A. Holes act as a positive charge B. Holes can be bound to an atomic nucleus C. Holes are repelled by electrons D. Holes have a drift velocity Q6. Which of the following would best describe the material composing a p-type semiconductor? A. Extremely pure silicon with some of the silicon electrons removed leaving holes in the resulting crystal lattice B. Extremely pure silicon with a certain number of extra electrons added leaving some free electrons in the crystal lattice C. Pure silicon that has small amounts of an element that has one less valence electron D. Pure silicon that has small amounts of an element that has one more valence electron "6 45 3" -* " /NVM UJ NF EJ B G P S Q I Z T J D T T U V E F O U T 4 of 10 XXX ITDQIZTJDT FEVBV Extended Answer Q7. As temperature decreases, how does the resistance to the flow of electrons in good conductors compare with the resistance to the flow of electrons in semiconductors? Use diagrams where appropriate. Q8. In terms of semiconductors, explain the concept of electrons and holes. "6 45 3" -* " /NVM UJ NF EJ B G P S Q I Z T J D T T U V E F O U T 5 of 10 XXX ITDQIZTJDT FEVBV Q9. In terms of band structures and relative electrical resistance, describe the differences between a conductor, an insulator, and a semiconductor. Use diagrams where appropriate. Q10. List 5 examples of how semiconductors have been applied in technology. "6 45 3" -* " /NVM UJ NF EJ B G P S Q I Z T J D T T U V E F O U T 6 of 10 XXX ITDQIZTJDT FEVBV Semiconductors – Cloze passage The concepts of semiconductors are a fundamentally important aspect of physics. In the last __________ years, semiconductors have revolutionised our lives, from the size of personal computers to the introduction use of plasma and __________ screen TVs. To understand semiconductors, we first have to consider a single __________. In a single atom, the energies of electrons are __________. This means they exist at specific energy levels. No electron may remain between these energy levels, and these energy values are called forbidden energy gaps. If there is no disturbance, such as an electric field, the __________ will fill all the energy levels from the ground up. When two __________ atoms are brought into close proximity, their energy levels shift with respect to one and other. In a solid vast numbers of atoms are closely packed together. The interaction of the atoms causes splitting and merging of energy levels into __________. Each band is composed of an enormous - but __________ - number of energy levels. These bands are separated by __________. If every __________ within an energy band is filled, then the band is filled. If all the energy bands are either completely filled or completely empty then no electrons can __________ in an electric field. If an energy band is partially filled, then it is __________ active. All __________ in insulators are either completely filled or completely empty. Conductors always have one __________ energy band. ___________ have partially filled energy bands when they are heated. Imagine heating up a Tungsten filament, which is a conductor. Initially the resistance is quite low but as we heat it up the resistance __________. This is due to increased thermal energy and __________ between the electrons and the metal ions within the lattice. For the semiconductor, imagine heating up a Germainium rod. Initially, the resistance is quite __________ but as we heat it up the resistance __________. This is because we are supplying the electrons with enough energy to cross the energy gap from the valence band into the conduction band. For the __________, imaging heating a piece of rubber tubing. The resistance is so __________ that a multimeter cannot display the result. This is because the energy gap between the valence band and conduction band is just so large in __________. We can change the number of valance electrons around each atom by replacing one of the semiconductor atoms with a different atom. This process is called __________. Materials that become semiconductors through __________ are called extrinsic semiconductors. Semiconductors are doped with either an atom from group III or group V, depending on the properties required. Doping with a __________ atom results in a missing electron in the lattice because group III atoms have one less __________ than group IV atoms. This missing electron is considered to be a region of missing negative charge in the valance band, and is called a hole. This type of doping is called positive-type, or __________ doping. Doping with a __________ atom results in an extra electron being inserted into the lattice. This is because group V atoms have one more electron in their valance band than group IV atoms. The presence of this extra electron means there is excess __________ carriers around the lattice. Therefore, this type of semiconductor being called a negative-type, or __________ semiconductor. "6 45 3" -* " /NVM UJ NF EJ B G P S Q I Z T J D T T U V E F O U T 7 of 10 XXX ITDQIZTJDT FEVBV Word List: Atom High Group V P-type Energy bands Valence electron Identical LCD Finite Semiconductors Fifty Decreases Electrically Doping Collisions Insulators Move Quantised N-type Doping Partially filled Forbidden gaps Increases Electrons Group III Insulator Energy bands Negative charge Energy level High "6 45 3" -* " /NVM UJ NF EJ B G P S Q I Z T J D T T U V E F O U T 8 of 10 XXX ITDQIZTJDT FEVBV Semiconductors – A discussion of different explanations In senior textbooks there are two common models for conduction. The biggest difference between the two models is whether they can explain the change in resistance with temperature. This resource looks at the physical reason for energy bands and then discusses two models. It is intended for teachers or advanced students. What is an energy band? In a single atom, the energies of electrons are quantised. This means they exist at specific energy levels. Between these energy levels are forbidden energy gaps in which no electron may remain. If there is no disturbance, such as an electric field, the electrons will fill all the energy levels from the ground up. When two identical atoms are brought into close proximity, their energy levels shift with respect to one and other. For a solid, vast numbers of atoms are packed closely together. The interaction of all the atoms causes splitting and merging of energy levels into energy bands. Each energy band is composed of an enormous - but finite - number of energy levels. If every energy level within an energy band is filled then no additional electrons can take on those energy values. These bands are separated by band gaps, or forbidden energy levels. Model I This is a model of conduction commonly found in textbooks: In insulators the valence band is always full, while the conduction band is always empty. The energy gap between the valence band and the conduction band is too large to be overcome by thermally exciting the electrons. For conductors, such as metals, the conduction and valence bands overlap. Thus valence electrons easily gain enough energy to become available for conduction. In semiconductors the energy gap between the conduction band and the valence band is very small – usually less than 1 eV. Electrons can gain sufficient energy when heated or exposed to light to be promoted to the conduction band. Silicon and germanium are two common semiconductors. When temperatures are low, the electrons do not have enough energy to be in the conduction band, so the material acts as an insulator. At higher temperatures, the valence band is nearly full and the conduction band is slightly full. This explanation often has a diagram similar the following: "6 45 3" -* " /NVM UJ NF EJ B G P S Q I Z T J D T T U V E F O U T 9 of 10 XXX ITDQIZTJDT FEVBV Problems with model I This model correctly explains semiconductors and insulators. However, it does not explain the temperature dependence of resistance in conductors. As temperature decreases electrons lose energy. In this model, the electron would thus drop from the conduction band into the valence band. This would decrease conduction. This is not what happens in practice. In practice, conduction increases as temperature decreases. This is because at lower temperatures there is less electron scattering from the lattices. Model II In solids where all the energy bands are either completely filled or completely empty then no electrons can move in an electric field. Insulators have completely filled or empty energy bands at all temperatures. Semiconductors have completely filled or empty energy bands at low temperatures. If an energy band is partially filled, then it is electrically active. Semiconductors have partially filled energy bands when they are heated. Conductors always have a partially filled energy band, between 10 and 90 percent – depending on the material. When electrons gain kinetic energy, through increased temperature or the presence of an electric field, the electron occupies a higher allowed energy within the same band. This is shown in the following diagram. This representation remains the same for conductors and insulators at all temperatures. "6 45 3" -* " /NVM UJ NF EJ B G P S Q I Z T J D T T U V E F O U T 10 of 10 XXX ITDQIZTJDT FEVBV Superconductors – Lesson Outline Syllabus References 9.4.4.2.6 - Discuss the BCS theory 9.4.4.2.7 - Discuss the advantages of using superconductors and identify limitations to their use 9.4.4.3.3 – Analyse information to explain why a magnet is able to hover above a superconducting material that has reached the temperature at which it is superconducting 9.4.4.3.4 - Gather and process information to describe how superconductors and the effects of magnetic fields have been applied to develop a maglev train Resources Video: Meissner Effect http://www.hscphysics.edu.au/resource/Meissner.flv Video: Superconductor Theory http://www.hscphysics.edu.au/resource/Supertheory.flv Video: Superconductor Applications http://www.hscphysics.edu.au/resource/8n98w83hfvm9356z Pre-video Activities Split students into groups of four, hand out butcher’s paper and markers. Students draw a concept map with the following words: Metals Lattice Vibrations Electron Resistance Temperature Conduction View Video Video: Meissner Effect http://www.hscphysics.edu.au/resource/Meissner.flv Activities With students in the same groups of four, hand out blank butcher’s paper. Students model what was happening in the video using physics principles they know. They then check if they were right in the following theory video. "6 45 3 " -*"/NVMU J NFE JB G P S Q I Z T J D T T U V E F O U T 1 of 7 XXX ITDQIZTJDT FEVBV View Video Video: Superconductor Theory http://www.hscphysics.edu.au/resource/Supertheory.flv Activities Students model the Meissner Effect again, this time using principles they learnt about in the video. Students add to their concept map, using their own words. In groups, students compare the model for the conduction of electricity in metals at room temperature with the model for conduction of electricity in superconductors below the critical temperature. Encourage the use of diagrams and tables. Representatives present the group’s responses to the rest of the class. Encourage class discussion and questioning. Students then brainstorm potential applications of superconductivity. Then students watch a video with a physics researcher discussing applications. View Video Video: Superconductor Applications http://www.hscphysics.edu.au/resource/8n98w83hfvm9356z Post-video Activities Post-video Activity: Concepts in Superconductivity quiz Students complete the quiz individually. Advanced students read through the discussion about different explanations for superconductors. "6 45 3 " -*"/NVMU J NFE JB G P S Q I Z T J D T T U V E F O U T 2 of 7 XXX ITDQIZTJDT FEVBV Superconductors – Concepts Q1. The resistance of mercury at various temperatures is shown in the graph. Between which two temperatures does mercury always act as a superconductor? A. B. C. D. O K and 4.2 K 4.2 K and 4.5 K 4.5 K and 8.0 K 0K and 8.0 K Q2. Which of the following graphs show the behaviour of a superconducting material? "6 45 3 " -*"/NVMU J NFE JB G P S Q I Z T J D T T U V E F O U T 3 of 7 XXX ITDQIZTJDT FEVBV Extended Answer Q3. The following image shows a magnet hovering above a superconducting disk. Explain why the magnet is able to hover above the superconductor. Q4. Some materials become superconductors when cooled to extremely low temperatures. Identify THREE properties of superconductors. "6 45 3 " -*"/NVMU J NFE JB G P S Q I Z T J D T T U V E F O U T 4 of 7 XXX ITDQIZTJDT FEVBV Q5. Explain how superconductivity can be modelled according to the BCS theory and the consequences for technology. Q6. There are a few areas in which energy savings can be made by the use of superconductors. One of these is electricity generation and transmission. Discuss how energy savings can be achieved in this area. Q7. Identify limitations of superconductors in technology. "6 45 3 " -*"/NVMU J NFE JB G P S Q I Z T J D T T U V E F O U T 5 of 7 XXX ITDQIZTJDT FEVBV Superconductors – A discussion of different explanations There are a number of explanations that can be given to students to help them visualize and understand superconductivity. This resource discusses a few of these explanations and is intended for teachers and advanced students. Standard Explanation Electrons are able to move through a conductor without feeling resistance because they form Cooper pairs. The lattice does not have enough thermal energy to scatter the pairs. The electrons form a Cooper pair through an interaction with the crystal lattice. As one electron moves through the lattice, the lattice ions are attracted to the electron and so move closer to the electron. This creates a region of excess positive charge. Another electron will be attracted to this region, and thus move towards to first electron. This attraction through the lattice forms the Cooper pair bond and the electrons then move through the lattice undisturbed. However, there is a significant problem with this explanation in terms of conservation of momentum. There is another explanation that accounts for momentum. Advanced Explanation An electron is travelling along and collides with the lattice. The lattice absorbs some of the electron’s momentum. This momentum propagates as a wave in the lattice called a phonon. A phonon is a quantised (local) lattice vibration. The phonon travels through the lattice until a while down the track it bumps into a second electron. The momentum is transferred to this second electron, which then experiences a push forward. As photons are quantised particles, all the momentum must be transferred. The lattice is therefore facilitating the bond between the first and second electron. As the lattice does not end up keeping any of the momentum, the electron pair has not lost any energy to the lattice and therefore has not felt any resistance. This momentum exchange obeys the conservation of momentum argument. However, from this explanation, it is difficult to explain the origins of the critical temperature for the superconducting material. The inadequacy of this explanation is a result of the lack of considerations of quantum effects. These effects as discussed further in the technical explanation. Analogy Here is analogy which better complies with the physics. In Naples, Italy there is an ingenious train called the funicular, which runs up and down a steep slope. Basically, a cable has the cars attached to it, and the cable runs on a loop around wheels at the top and bottom of the slope. As one car goes down, the other goes up, and the two exchange potential energy and form a pair with total momentum zero. This way, the motor driving the funicular does very little work. The Cooper pair is like a pair of cars on a funicular, in that each electron in the pair helps the other move more easily through the lattice. They have total momentum zero as well, but work better than the funicular in one way: no energy is expended as they move along, since they have too little momentum as a pair to lose energy by giving energy and momentum to the lattice in the form of phonons. "6 45 3 " -*"/NVMU J NFE JB G P S Q I Z T J D T T U V E F O U T 6 of 7 XXX ITDQIZTJDT FEVBV Technical Explanation The technical explanation that best matches experimental evidence has been formulated using quantum physics. A key conceptual element in this theory is the pairing of electrons close to the Fermi level into Cooper pairs through interaction with the crystal lattice. This pairing results from a slight attraction between the electrons related to lattice vibrations; the coupling to the lattice is called a phonon interaction. The lattice absorbs and reemits momentum within the uncertainty time determined by Heisenberg’s uncertainty principle, thus it can be considered a virtual phonon. This can happen over 100’s of lattice spacings and the pairs are constantly breaking and reforming. However, as they are non-distinguishable, it is useful to think of a pair as constantly bound. Pairs of electrons can behave very differently from single electrons, which are fermions and must obey the Pauli exclusion principle. The pairs of electrons act more like bosons, which can condense into the same energy level. However, to form this boson pair, they must have opposite angular momentums or k-vectors due to the Pauli exclusion principle. The electron pairs have a slightly lower energy and leave an energy gap above them on the order of 0.001 eV, which inhibits the kind of collision interactions that lead to ordinary resistivity. For temperatures such that the thermal energy is less than the band gap, the material exhibits zero resistivity. The interaction is represented in the following diagram below (based on a Feynman diagram). Key Points ! Electrons are linked indirectly by phonons ! They have the opposite momentum ! The paired electrons may by hundreds of lattice spacings apart. ! The pairs are constantly breaking and forming different pairs. ! The pairs of electrons act more like bosons, which can condense into the same energy level. ! The electron pairs have a slightly lower energy and leave an energy gap above them on the order of 0.001 eV, which inhibits the kind of collision interactions, which lead to ordinary resistivity. ! It is a quantum effect. "6 45 3 " -*"/NVMU J NFE JB G P S Q I Z T J D T T U V E F O U T 7 of 7 XXX ITDQIZTJDT FEVBV Planck & Einstein – Lesson Outline Syllabus Reference 9.4.2.3.5 – Process information to discuss Einstein and Planck’s differing views about whether science research is removed from social and political forces. Resources Video: Planck & Einstein http://www.hscphysics.edu.au/resource/PlaEin.flv Pre-video Activities Divide students into groups of 4-5 then give each group a piece of A3 paper and some makers. Each group brainstorm either the ways science has affected society or the ways society has influenced science. One representative presents the group’s work to the rest of the class. Encourage class discussion and questioning. Collate all groups’ responses on board or screen. Lead discussion with the question: Is science and science research therefore removed from social and political forces? Pre-video resource: A chronology of significant event Pre-video resource: Other important dates Students are given the resources: A chronology of significant events and Other important dates. Students construct a scaled timeline incorporating all the information. Students should work in small groups and be provided with butcher’s paper - they should make an effort to differentiate events into groups: Planck, Einstein, Social Political and Scientific Discoveries. Allow the student to think of creative ways to do this, but ensure groups are clearly distinguished and that the timeline is clearly scaled. A layout suggestion would be columns encompassing each group with a common timeline to the left or right. View Video Video: Planck & Einstein http://www.hscphysics.edu.au/resource/PlaEin.flv "6 45 3 " -* "/NV MU J NFE JB G P S Q I Z T J D T T U V E F O U T 1 of 4 XXX ITDQIZTJDT FEVBV Post-Video Activities Students divided up into groups of 3-4. Some groups take Planck, others take Einstein. Groups compare the actions of Planck or Einstein and the social/political view that such an action suggested. One representative presents the group’s work to the rest of the class. Encourage class discussion and questioning. Collate all groups’ responses on board or screen. Pose the syllabus question to the class again. Initiate a class discussion on the dot point and what was learnt during the course of the lesson. Give students time to write their own summary on the dot point and lesson for further study. An optional research task, in the library or a computer room, assign pairs of students to investigate questions such as: Did Germany ever build an atomic bomb? The facts behind Planck’s anti-Semitism Which other Jewish scientists were affected by Nazi rule in Germany? Did Planck push for an atomic bomb? Why did Einstein leave Germany? What did Einstein and Planck do in WW1? How were Planck and Einstein seen by their colleagues? Why did Einstein settle in the United States? Did Einstein work on the Manhattan project? Which famous physicists worked on the Manhattan project? Pairs should then report back to the whole class with their findings. "6 45 3 " -* "/NV MU J NFE JB G P S Q I Z T J D T T U V E F O U T 2 of 4 XXX ITDQIZTJDT FEVBV Planck & Einstein– Other Important Dates 1831 - Michael Faraday discovers electromagnetic induction 1859 - Charles Darwin publishes On the Origin of Species, the foundation of evolution 1884 - James Clerk Maxwell’s publishes 4 equations which explain all of electromagnetism and proposing the idea of electromagnetic waves, with light being such a wave 1886 - Heinrich Hertz conducts his ‘spark gap’ experiment, discovering the photoelectric effect, and also producing the first experimental evidence for Maxwell’s electromagnetic waves. 1887 - The Michelson Morley experiment to detect the ‘aether wind’ 1897 - J.J. Thompson’s discovers the electron, and determines its charge/mass ratio 1900 - Around this period, Phillip Lenard investigates experimentally the behaviour of the photoelectric effect. 1901 - Australian federation 1918 - Ernest Rutherford discovers the proton 1927 - Neils Bohr and Werner Heisenberg put forward the Copenhagen interpretation of Quantum mechanics 1932 - James Chadwick discovers the neutron 1936 - In Britain first ever broadcast television 1938 - Otto Hahn and Fritz Strassmann ‘split’ the uranium atom in a German university identifying nuclear fission. Their Jewish associate Lise Meitner, integral in this discovery had fled to Sweden earlier in the year to escape Nazi persecution 1913 - Bohr’s proposes his model of the atom (combining quantum theory with Rutherford’s 1911 model, it is often called the Rutherford-Bohr model) 1941 - The Japanese attack Pearl Harbour, bring the United States into WWII 1950 - In the United States, the first colour television (Australia 1975) 1953 - Watson and Crick publish the double helical model of DNA 1958 - The first silicon chip (an integrated circuit, using transistor technology) 1961 - Russian cosmonaut Yuri Gagarin first man in space. 1969 - Neil Armstrong from Apollo 11 becomes the first man to land on the moon "6 45 3 " -* "/NV MU J NFE JB G P S Q I Z T J D T T U V E F O U T 3 of 4 XXX ITDQIZTJDT FEVBV Planck & Einstein– A Chronology of Important Dates 1858 1879 Max Planck born in Kiel, Germany 1892 1896 Planck becomes professor of theoretical physics at the University of Berlin 1900 Albert Einstein born in Ulm, Germany Einstein renounces German citizenship, in opposition to German militarism, remains stateless until 1901 when granted Swiss citizenship Planck publishes his famous account of the problem of blackbody radiation, proposing his own formula for the radiation of energy (E= hf), the foundation of Einstein graduates from ETH, and takes a job at the Swiss patent office 1905 Einstein publishes three groundbreaking papers on the photoelectric effect, special relativity and Brownian motion !"#$%&!'#&(&)*+,-.C&8*#9'-5&+#*'0$5&'33*;0*%& 1914 1915 1918 1919 1921 1930 1933 Planck signs the Manifesto of the Ninety-three Intellectuals supporting German military. Einstein signs a counter manifesto. Einstein first publishes the definitive version of his theory of General Relativity Planck receives the Nobel Prize in Physics for his work on energy radiation !"#$%&!'#&(&*-%.&/,01&8*#9'-5H.&.,+-,-+&"3&01*&E#*'05&"3&I*#.',$$*.&& Einstein receives the Nobel Prize in Physics for the Photoelectric effect Planck appointed as president of the Kaiser Wilhelm Institute 8*#9'-5&)*;"9*.&'&6'7,&%,;0'0"#.1,<C&& :%"$3&=,0$*#&'<<",-0*%&D1'-;*$$"#&"3&8*#9'-5& Planck meets with Hitler in an attempt to influence policy change in regards to the welfare of his Jewish scientist colleagues. Einstein resigns from the Kaiser Wilhelm institute, and takes up a professorship at Princeton in the United States 1937 Planck resigns as president of the Kaiser Wilhelm Institute in protest against Nazi policy 1939 !"#$%&!'#&((&)*+,-.&/,01&01*&,-2'.,"-&"3&4"$'-%&)5&6'7,&8*#9'-5& Einstein first writes to President Roosevelt, warning of the potential for a German atomic bomb and urging the United States to develop one first 1942 1945 1947 1947 1955 E1*&"33,;,'$&F'-1'00'-&4#"G*;0C&0"&%*2*$"<,-+&01*&'0"9,;&)"9)C&)*+,-.& Planck’s son, advisor and close friend, Erwin, dies at the hands of the Gestapo after being implicated in an assassination attempt on Hitler in 1944, destroying Planck’s will to live :0"9,;&)"9).&%#"<<*%&"-&=,#".1,9'&'-%&6'+'.'>,&)5&01*&?-,0*%&@0'0*.& !"#$%&!'#&((&*-%.&/,01&01*&.A##*-%*#&"3&B'<'-& Planck saves the Kaiser-Wilhelm Society from post-WWII disbandment Planck dies on the 4th of October, in Göttingen, Germany, aged 89 Einstein dies on the 19th of April, at Princeton, New Jersey, aged 76 "6 45 3 " -* "/NV MU J NFE JB G P S Q I Z T J D T T U V E F O U T 4 of 4