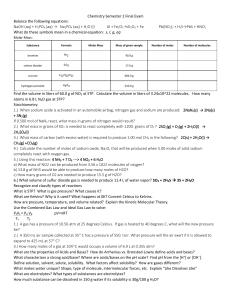
L1 – CHEMISTRY FINAL REVIEW
... 32. Describe the Van Der Waals forces between water molecules called dipole interactions or Hydrogen bonds. H-bond is a strong intermolecular bond between the slightly positive Hydrogen end of one water molecule and the slightly neg. oxygen end of an adjacent water molecule. 33. Name 4 unique proper ...
... 32. Describe the Van Der Waals forces between water molecules called dipole interactions or Hydrogen bonds. H-bond is a strong intermolecular bond between the slightly positive Hydrogen end of one water molecule and the slightly neg. oxygen end of an adjacent water molecule. 33. Name 4 unique proper ...
The Actual Speed Limit for Particles with Rest Mass Not Equal to
... mass that any finite rest mass can achieve is not infinite but finite. However, the actual mass-limit for any rest-mass body is not given by or isn’t the relativistic mass-limit. This actual mass-limit is beyond relativistic mass-limit to infinity .i.e. relativistic mass-limit is practically attaina ...
... mass that any finite rest mass can achieve is not infinite but finite. However, the actual mass-limit for any rest-mass body is not given by or isn’t the relativistic mass-limit. This actual mass-limit is beyond relativistic mass-limit to infinity .i.e. relativistic mass-limit is practically attaina ...
Curriculum Map for: Regents Physics - Scotia
... proportional to the speed of a wave.* 4.3k All frequencies of electromagnetic radiation travel at the same speed in a vacuum.* 4.3l Diffraction occurs when waves pass by obstacles or through openings. The wavelength of the incident wave and the size of the obstacle or opening affect how the wave ...
... proportional to the speed of a wave.* 4.3k All frequencies of electromagnetic radiation travel at the same speed in a vacuum.* 4.3l Diffraction occurs when waves pass by obstacles or through openings. The wavelength of the incident wave and the size of the obstacle or opening affect how the wave ...
On the Essence of Electric Charge
... The Standard Model of elementary particles, despite its successes, fails to derive and calculate the radii and masses of the elementary particles, and fails to explain what charge is. In this paper we present a new understanding of the essence of electric charge. We show that charge is not alien to ...
... The Standard Model of elementary particles, despite its successes, fails to derive and calculate the radii and masses of the elementary particles, and fails to explain what charge is. In this paper we present a new understanding of the essence of electric charge. We show that charge is not alien to ...
Trajectory-Wave Approach to Electron Dynamics in Hydrogen Atom
... occur along a trajectory the presence of which is a reflection of the existence of a particle, as well as it is assumed that any motion is defined by a wave V(x,t). It is assumed that there is an explicit relationship between the trajectory and wave equations of the electron, which are established o ...
... occur along a trajectory the presence of which is a reflection of the existence of a particle, as well as it is assumed that any motion is defined by a wave V(x,t). It is assumed that there is an explicit relationship between the trajectory and wave equations of the electron, which are established o ...
Data: I am writing out the question and underlining it.
... Well when fossil fuels are burned, or maybe even things like wood or who knows, scientists most likely calculate the molecules given off so they can come up with these statistics. Well maybe they deal with moles or liters of gas at STP, who knows, but I’m sure somewhere in there scientists will have ...
... Well when fossil fuels are burned, or maybe even things like wood or who knows, scientists most likely calculate the molecules given off so they can come up with these statistics. Well maybe they deal with moles or liters of gas at STP, who knows, but I’m sure somewhere in there scientists will have ...
The Bohr model
... achieved through quantization of the energy released by atoms was achieved by Niels Bohr. The model had the following properties, as summarized by Bohr [2, 3]: (1) The electron emits radiation when transitioning from one discreet state to the next. (2) Classical mechanics is valid when the electron ...
... achieved through quantization of the energy released by atoms was achieved by Niels Bohr. The model had the following properties, as summarized by Bohr [2, 3]: (1) The electron emits radiation when transitioning from one discreet state to the next. (2) Classical mechanics is valid when the electron ...
Work Function of Metals: Correlation Between Classical Model and
... In gases (ex., Bohr model), the above definition is usually rigorously applied, whereas in solids there are numerous different definitions of the work function which vary widely, imprecisely and incorrectly. (1.1) Ionization Energy of Gaseous Atoms. The key feature of the well known Bohr model is st ...
... In gases (ex., Bohr model), the above definition is usually rigorously applied, whereas in solids there are numerous different definitions of the work function which vary widely, imprecisely and incorrectly. (1.1) Ionization Energy of Gaseous Atoms. The key feature of the well known Bohr model is st ...
Chapter 18: The Representative Elements The Representative
... Lose their valence e- easily (great reducing agents). Most violently reactive of all the metals. React strongly with H2O(l); the vigor of the reaction increases down the group. The alkali metals are all too easily oxidized to be found in their free state in nature. ...
... Lose their valence e- easily (great reducing agents). Most violently reactive of all the metals. React strongly with H2O(l); the vigor of the reaction increases down the group. The alkali metals are all too easily oxidized to be found in their free state in nature. ...
Chapter 18: The Representative Elements
... this group to straddle between metal and non metal. The heavier elements of the group are more likely to keep their s electrons and can have oxidation numbers of +2 or +4. ...
... this group to straddle between metal and non metal. The heavier elements of the group are more likely to keep their s electrons and can have oxidation numbers of +2 or +4. ...
Chapter 9 PPT
... The time interval during which the velocity changes from its initial to final values is assumed to be short The interaction forces are assumed to be much greater than any external forces present ...
... The time interval during which the velocity changes from its initial to final values is assumed to be short The interaction forces are assumed to be much greater than any external forces present ...
AP Chemistry
... Which best helps to account for the net loss of entropy? (D) verify the mass of water that was cooled. (A) Cl- ions are much larger in size than Ca2+ ions. (B) The particles in solid calcium chloride are more ordered than are particles in amorphous solids. (C) Water molecules in the hydrated Ca2+ an ...
... Which best helps to account for the net loss of entropy? (D) verify the mass of water that was cooled. (A) Cl- ions are much larger in size than Ca2+ ions. (B) The particles in solid calcium chloride are more ordered than are particles in amorphous solids. (C) Water molecules in the hydrated Ca2+ an ...
Electrostatic-directed deposition of nanoparticles on a field
... the electrostatic, van der Waals, and image forces, was used to explain the observed results. (Some figures in this article are in colour only in the electronic version) 1. Introduction Functional nanoparticles have been widely considered as the building blocks of potential micro- and nano-scale ele ...
... the electrostatic, van der Waals, and image forces, was used to explain the observed results. (Some figures in this article are in colour only in the electronic version) 1. Introduction Functional nanoparticles have been widely considered as the building blocks of potential micro- and nano-scale ele ...
File
... • The following reaction shows table salt production. How many moles of sodium chloride are produced from 0.02 moles of chlorine? ...
... • The following reaction shows table salt production. How many moles of sodium chloride are produced from 0.02 moles of chlorine? ...
PPT
... Using Wick’s decompositions at 2-particle level or 3-particle level show similar results. ...
... Using Wick’s decompositions at 2-particle level or 3-particle level show similar results. ...
qp13 - Smart Edu Hub
... 18 The graph shows how the pH changes as an acid is added to an alkali. acid + alkali → salt + water Which letter represents the area of the graph where both acid and salt are present? ...
... 18 The graph shows how the pH changes as an acid is added to an alkali. acid + alkali → salt + water Which letter represents the area of the graph where both acid and salt are present? ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.