Mathematics of Quantum Mechanics
... any given time. Werner Heisenberg’s uncertainty principle, or the Copenhagen interpretation of quantum mechanics, encapsulates this idea: One can never know with perfect accuracy both of those two important factors which determine the movement of one of the smallest particles—its position and its ve ...
... any given time. Werner Heisenberg’s uncertainty principle, or the Copenhagen interpretation of quantum mechanics, encapsulates this idea: One can never know with perfect accuracy both of those two important factors which determine the movement of one of the smallest particles—its position and its ve ...
C1 – Topic 2 notes - ARK Elvin Academy
... contains and the ratios in which their atoms are present E.g with calcium carbonate: o Its chemical formula is CaCO3 o It’s a compound o It contains 3 elements: calcium, carbon and oxygen o It contains 1 calcium atom, 1 carbon atom and 3 oxygen atoms Balancing equations: This involves first writing ...
... contains and the ratios in which their atoms are present E.g with calcium carbonate: o Its chemical formula is CaCO3 o It’s a compound o It contains 3 elements: calcium, carbon and oxygen o It contains 1 calcium atom, 1 carbon atom and 3 oxygen atoms Balancing equations: This involves first writing ...
The Story of Nuclear Matter
... much more complex than the electromagnetic force operating in atomic phenomena. The force between two nucleons (the so-called Strong force) , obtained by studying the scattering of nucleons off one another, consists of several terms whose dependence on distance is a complicated function, unlike the ...
... much more complex than the electromagnetic force operating in atomic phenomena. The force between two nucleons (the so-called Strong force) , obtained by studying the scattering of nucleons off one another, consists of several terms whose dependence on distance is a complicated function, unlike the ...
Topic 2 notes - WordPress.com
... contains and the ratios in which their atoms are present E.g with calcium carbonate: o Its chemical formula is CaCO3 o It’s a compound o It contains 3 elements: calcium, carbon and oxygen o It contains 1 calcium atom, 1 carbon atom and 3 oxygen atoms Balancing equations: This involves first writing ...
... contains and the ratios in which their atoms are present E.g with calcium carbonate: o Its chemical formula is CaCO3 o It’s a compound o It contains 3 elements: calcium, carbon and oxygen o It contains 1 calcium atom, 1 carbon atom and 3 oxygen atoms Balancing equations: This involves first writing ...
Nuclear lattice model and the electronic configuration
... filled in first with the electrons and only then the orbitals of high energy are filled.”. The energy levels calculated from the hydrogenic model, or the main quantum number follows the sequence of 2n2. Only the first two entries of this quantum number sequence is consistent with the periodic sequen ...
... filled in first with the electrons and only then the orbitals of high energy are filled.”. The energy levels calculated from the hydrogenic model, or the main quantum number follows the sequence of 2n2. Only the first two entries of this quantum number sequence is consistent with the periodic sequen ...
Final Spring 2011 with solutions
... When calculating numerical values, be sure to keep track of units. You may use this exam or come up front for scratch paper. Be sure to put a box around your final answers and clearly i ...
... When calculating numerical values, be sure to keep track of units. You may use this exam or come up front for scratch paper. Be sure to put a box around your final answers and clearly i ...
Electrostatic Forces and Fields
... electron (a negative electric charge), and the neutron (a zero electric charge.) These elementary particles are the building blocks for all matter and typical atomic structure as revealed by experiments has a heavy dense nucleus (composed of protons and neutrons) surrounded by electrons in specific ...
... electron (a negative electric charge), and the neutron (a zero electric charge.) These elementary particles are the building blocks for all matter and typical atomic structure as revealed by experiments has a heavy dense nucleus (composed of protons and neutrons) surrounded by electrons in specific ...
EXAM3
... Physics 111 PRACTICE Final Exam, Spring 2013, Version B STUDENTS: PLEASE NOTE that this practice exam is only a small selection of possible problems which could be on the final. While all of these problems would be ‘fair game’ for the final exam, there will be problems on the final exam which are NO ...
... Physics 111 PRACTICE Final Exam, Spring 2013, Version B STUDENTS: PLEASE NOTE that this practice exam is only a small selection of possible problems which could be on the final. While all of these problems would be ‘fair game’ for the final exam, there will be problems on the final exam which are NO ...
Modification of the Strong Nuclear Force by the
... …where E is energy, t is time, x is position, p is momentum and h is Planck’s constant. Thus the Heisenberg Uncertainty Principle sets a fundamental limit on the precision with which these conjugate quantities are allowed to be determined. Now if we work out the quantum version of a simple mechanica ...
... …where E is energy, t is time, x is position, p is momentum and h is Planck’s constant. Thus the Heisenberg Uncertainty Principle sets a fundamental limit on the precision with which these conjugate quantities are allowed to be determined. Now if we work out the quantum version of a simple mechanica ...
Word Doc - Bodge It and Scarper Ltd
... momentum of what is in the box using an external sensor, we can of course deduce it by moving the box about but that is not what I mean here by “from outside the box”. We can sense magnetic and electric fields that escape from the box easily but not spin. We couldn’t measure magnetic and electric f ...
... momentum of what is in the box using an external sensor, we can of course deduce it by moving the box about but that is not what I mean here by “from outside the box”. We can sense magnetic and electric fields that escape from the box easily but not spin. We couldn’t measure magnetic and electric f ...
Symmetry and statistics
... As is well known (see Chapter 14), such a form is dictated by the requirement that it should be possible to re-parametrize the electron wave function by an arbitrary phase factor, with time- and space-dependent phase f (r, t), as ψ(r, t) → ei f (r,t) ψ(r, t). Even the necessity of the existence of t ...
... As is well known (see Chapter 14), such a form is dictated by the requirement that it should be possible to re-parametrize the electron wave function by an arbitrary phase factor, with time- and space-dependent phase f (r, t), as ψ(r, t) → ei f (r,t) ψ(r, t). Even the necessity of the existence of t ...
Molekylfysik - Leiden Institute of Physics
... Direction of the transmitted wave is (Left Right): B’=0 since B’e-ikx is a wave travelling in the (Right Left) direction. The wave function must be continuous at the edges of the barrier (for x=0 and L): (1) For x=0: (x<0)= (0
... Direction of the transmitted wave is (Left Right): B’=0 since B’e-ikx is a wave travelling in the (Right Left) direction. The wave function must be continuous at the edges of the barrier (for x=0 and L): (1) For x=0: (x<0)= (0
Blueshift of the surface plasmon resonance in silver nanoparticles
... density profile in this local-response model, we follow the approach of Ref. [16] and assume that the free electrons move in an infinite spherical potential well. The approach just outlined of a local-response theory with an inhomogeneous electron density is very similar to the theoretical model use ...
... density profile in this local-response model, we follow the approach of Ref. [16] and assume that the free electrons move in an infinite spherical potential well. The approach just outlined of a local-response theory with an inhomogeneous electron density is very similar to the theoretical model use ...
Homework No. 09 (Spring 2016) PHYS 530A: Quantum Mechanics II
... with different electric charge: T3 = 1 (π + ), T3 = 0 (π 0 ), T3 = −1 (π − ). (Refer Table 1.) Consider the system of a nucleon and a pion. The electric charge of this system is Q = 21 + T3 . Check that a system of charge 2, T3 = 32 , is p+π + , according to the isospin assignments. Now, if the syst ...
... with different electric charge: T3 = 1 (π + ), T3 = 0 (π 0 ), T3 = −1 (π − ). (Refer Table 1.) Consider the system of a nucleon and a pion. The electric charge of this system is Q = 21 + T3 . Check that a system of charge 2, T3 = 32 , is p+π + , according to the isospin assignments. Now, if the syst ...
Two-orbital SU(N) magnetism with ultracold alkaline-earth
... Fermionic alkaline-earth atoms have unique properties that make them attractive candidates for the realization of atomic clocks and degenerate quantum gases. At the same time, they are attracting considerable theoretical attention in the context of quantum information processing. Here we demonstrate ...
... Fermionic alkaline-earth atoms have unique properties that make them attractive candidates for the realization of atomic clocks and degenerate quantum gases. At the same time, they are attracting considerable theoretical attention in the context of quantum information processing. Here we demonstrate ...
CHM 151LL: States of Matter: Physical and Chemical Changes
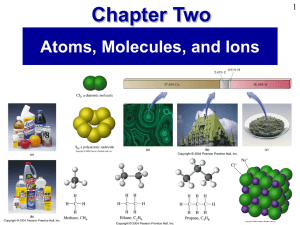
... able to classify a substance using these terms will help you identify the properties of the material. It helps to form a mental picture of a material so you can better understand how to describe it. For example, when someone says “atom”, you should visualize a single sphere. The word molecule means ...
... able to classify a substance using these terms will help you identify the properties of the material. It helps to form a mental picture of a material so you can better understand how to describe it. For example, when someone says “atom”, you should visualize a single sphere. The word molecule means ...
Atomic theory
In chemistry and physics, atomic theory is a scientific theory of the nature of matter, which states that matter is composed of discrete units called atoms. It began as a philosophical concept in ancient Greece and entered the scientific mainstream in the early 19th century when discoveries in the field of chemistry showed that matter did indeed behave as if it were made up of atoms.The word atom comes from the Ancient Greek adjective atomos, meaning ""uncuttable"". 19th century chemists began using the term in connection with the growing number of irreducible chemical elements. While seemingly apropos, around the turn of the 20th century, through various experiments with electromagnetism and radioactivity, physicists discovered that the so-called ""uncuttable atom"" was actually a conglomerate of various subatomic particles (chiefly, electrons, protons and neutrons) which can exist separately from each other. In fact, in certain extreme environments, such as neutron stars, extreme temperature and pressure prevents atoms from existing at all. Since atoms were found to be divisible, physicists later invented the term ""elementary particles"" to describe the ""uncuttable"", though not indestructible, parts of an atom. The field of science which studies subatomic particles is particle physics, and it is in this field that physicists hope to discover the true fundamental nature of matter.