Modified from College Physics, 8th Ed., Serway and Vuille. For the
... Every fundamental interaction is said to be mediated by the exchange of field particles. The electromagnetic interaction is mediated by the photon, the weak interaction by the W± and Z0 bosons, the gravitational interaction by gravitons, and the strong interaction by gluons. Section 30.4: Positrons ...
... Every fundamental interaction is said to be mediated by the exchange of field particles. The electromagnetic interaction is mediated by the photon, the weak interaction by the W± and Z0 bosons, the gravitational interaction by gravitons, and the strong interaction by gluons. Section 30.4: Positrons ...
Notes for the Structure of Atoms (Chapter 4, Sect
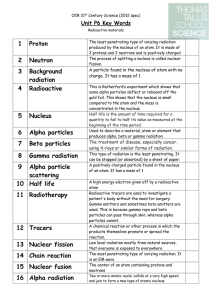
... a. alpha particles: positively charged and more massive than any others. Consists of protons and neutrons. Doesn’t travel far. b. beta particles: fast moving electrons (negatively charged) or positively charged particles (positrons). {neutrons decay to form protons and electrons, of which electrons ...
... a. alpha particles: positively charged and more massive than any others. Consists of protons and neutrons. Doesn’t travel far. b. beta particles: fast moving electrons (negatively charged) or positively charged particles (positrons). {neutrons decay to form protons and electrons, of which electrons ...
Chapter 2, section 4 Formation of Elements
... of the man-made isotopes are unstable. Unstable isotopes can become stable by releasing different types of particles. This process is called radioactive decay and the elements which undergo this process are called radioisotopes/. ...
... of the man-made isotopes are unstable. Unstable isotopes can become stable by releasing different types of particles. This process is called radioactive decay and the elements which undergo this process are called radioisotopes/. ...
File
... position of a single negatively charged particle in an atom and the particle's momentum cannot both be known at the same time. Scientists call this the "Heisenberg Uncertainty Principle." A common representation of this idea is to place the negatively charged particles in a cloud surrounding the nuc ...
... position of a single negatively charged particle in an atom and the particle's momentum cannot both be known at the same time. Scientists call this the "Heisenberg Uncertainty Principle." A common representation of this idea is to place the negatively charged particles in a cloud surrounding the nuc ...
Nuclear Chemistry – Chapter 25, chapter 4, section 4
... Nuclear chemistry is the study of the changes of the __________________ of atoms. ...
... Nuclear chemistry is the study of the changes of the __________________ of atoms. ...
The Atom - Effingham County Schools
... the observed properties of cathode rays that led to the discovery of the electron Summarize the experiment carried out by Rutherford and his co-workers that led to the discovery of the nucleus ...
... the observed properties of cathode rays that led to the discovery of the electron Summarize the experiment carried out by Rutherford and his co-workers that led to the discovery of the nucleus ...
Basic Atomic Theory
... • Atomic Number (Z) – The number of protons – OR the number of electrons ...
... • Atomic Number (Z) – The number of protons – OR the number of electrons ...
Element: pure substances that are made up of one kind of atom
... 7. _______________There are the same number of these two particles in an atom. 8. _______________The atomic number is the same as the number of these particles. 9. _______________The mass number is determined by which particles? ...
... 7. _______________There are the same number of these two particles in an atom. 8. _______________The atomic number is the same as the number of these particles. 9. _______________The mass number is determined by which particles? ...
HW4 - SMU Physics
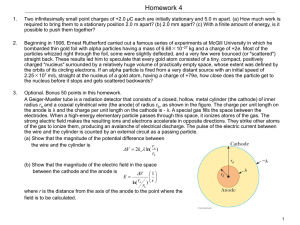
... Optional. Bonus 50 points in this homework. A Geiger-Mueller tube is a radiation detector that consists of a closed, hollow, metal cylinder (the cathode) of inner radius ra and a coaxial cylindrical wire (the anode) of radius rb, as shown in the figure. The charge per unit length on the anode is λ a ...
... Optional. Bonus 50 points in this homework. A Geiger-Mueller tube is a radiation detector that consists of a closed, hollow, metal cylinder (the cathode) of inner radius ra and a coaxial cylindrical wire (the anode) of radius rb, as shown in the figure. The charge per unit length on the anode is λ a ...
Atomic Structure Tick Sheet
... NUMBERS of positive protons and negative electrons so the charges cancel. I know that all atoms of the same element have the SAME number of protons. I know that atoms of DIFFERENT elements have DIFFERENT numbers of protons. I know that the ATOMIC NUMBER of an atom is the BOTTOM NUMBER next to the sy ...
... NUMBERS of positive protons and negative electrons so the charges cancel. I know that all atoms of the same element have the SAME number of protons. I know that atoms of DIFFERENT elements have DIFFERENT numbers of protons. I know that the ATOMIC NUMBER of an atom is the BOTTOM NUMBER next to the sy ...
Chapter 4 - Mr. Fischer.com
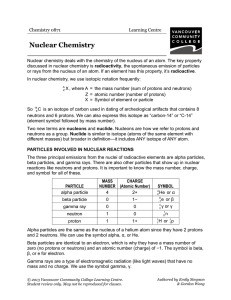
... proton is about 1840 times the mass of an electron. Protons are represented by p+. C. Neutrons are neutral particles and are located inside the nucleus. Neutrons have no effective charge and are therefore considered to be neutral. Neutrons have a mass of 1.67 x 10-24 g. Neutrons are represented by n ...
... proton is about 1840 times the mass of an electron. Protons are represented by p+. C. Neutrons are neutral particles and are located inside the nucleus. Neutrons have no effective charge and are therefore considered to be neutral. Neutrons have a mass of 1.67 x 10-24 g. Neutrons are represented by n ...
What is electricity
... Atoms contain particles that have charge Positively charged particle = proton Negatively charged particle = electron Neutral or particles with no charge are called neutrons Protons and Neutrons are found in the nucleus of the atom. ...
... Atoms contain particles that have charge Positively charged particle = proton Negatively charged particle = electron Neutral or particles with no charge are called neutrons Protons and Neutrons are found in the nucleus of the atom. ...
File
... Nuclear Binding Energy Nuclear binding energy is the energy required to disassemble a nucleus into free unbound neutrons and protons. Nuclear binding energy can be calculated from the difference of mass of a nucleus, and the sum of the masses of the number of free neutrons and protons that make up ...
... Nuclear Binding Energy Nuclear binding energy is the energy required to disassemble a nucleus into free unbound neutrons and protons. Nuclear binding energy can be calculated from the difference of mass of a nucleus, and the sum of the masses of the number of free neutrons and protons that make up ...
Atomic nucleus
The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The atomic nucleus was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.The diameter of the nucleus is in the range of 6985175000000000000♠1.75 fm (6985175000000000000♠1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 6986150000000000000♠15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.













![The atom: Structure (Grade 10) [NCS]](http://s1.studyres.com/store/data/015174795_1-33c5ca5b28bd2149af36b51eb860b44e-300x300.png)