Atomic Structure
... • 2. Atoms of the same element are identical. The atoms of one element are different from the atoms of another element. • 3. Atoms of different elements can physically mix together or can chemically combine in simplewhole number ratios to form compounds. • 4. Chemical reactions occur when atoms are ...
... • 2. Atoms of the same element are identical. The atoms of one element are different from the atoms of another element. • 3. Atoms of different elements can physically mix together or can chemically combine in simplewhole number ratios to form compounds. • 4. Chemical reactions occur when atoms are ...
Physical Science Week 1
... • Matter: Anything that takes up space and has mass. • Atom: The smallest unit of an element that maintains the properties of that element. ...
... • Matter: Anything that takes up space and has mass. • Atom: The smallest unit of an element that maintains the properties of that element. ...
Trends of the Periodic Table - Laureate International College
... o Electrons are added along the same energy level, increasing the attractive forces between protons and valence electrons requiring more IE to remove them. ...
... o Electrons are added along the same energy level, increasing the attractive forces between protons and valence electrons requiring more IE to remove them. ...
elementary particles history
... • Ernest Lawrence built his first successful cyclotron to provide acceleration in several small increments in 1930. ...
... • Ernest Lawrence built his first successful cyclotron to provide acceleration in several small increments in 1930. ...
Enrichment Opportunities: Atoms
... curiosity has shown us things smaller than anyone thought existed – first the atom and then subatomic particles. But scientists didn’t stop with protons, electrons, and neutrons. Instead, they devised sophisticated instruments called particle accelerators to take a close look at subatomic particles. ...
... curiosity has shown us things smaller than anyone thought existed – first the atom and then subatomic particles. But scientists didn’t stop with protons, electrons, and neutrons. Instead, they devised sophisticated instruments called particle accelerators to take a close look at subatomic particles. ...
THE ATOM Elements Isotopes Ions
... ELECTRON CONFIGURATION The electrons of an atom are not arranged randomly, but exist in shells. They are negatively charged but they do tend to pair up (much like girls going to the toilet!!! ☺). They can pair up because they spin on their axis and thus generate a magnetic field. Two electrons spin ...
... ELECTRON CONFIGURATION The electrons of an atom are not arranged randomly, but exist in shells. They are negatively charged but they do tend to pair up (much like girls going to the toilet!!! ☺). They can pair up because they spin on their axis and thus generate a magnetic field. Two electrons spin ...
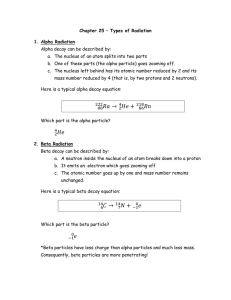
Chapter 25 – Types of Radiation 1. Alpha Radiation Alpha decay
... Positron decay is the mirror image of beta decay and can be described as: a. Something inside the nucleus breaks down causing a proton to become a neutron. b. It emits a positron which goes zooming off. c. The atomic number goes down by one and the mass number remains unchanged. Here is a typical po ...
... Positron decay is the mirror image of beta decay and can be described as: a. Something inside the nucleus breaks down causing a proton to become a neutron. b. It emits a positron which goes zooming off. c. The atomic number goes down by one and the mass number remains unchanged. Here is a typical po ...
of electrons - Midland ISD
... at a cake…all of the bullets (or alpha particles) would go straight through the cake (or gold foil atoms) ...
... at a cake…all of the bullets (or alpha particles) would go straight through the cake (or gold foil atoms) ...
Atomic nucleus
The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The atomic nucleus was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.The diameter of the nucleus is in the range of 6985175000000000000♠1.75 fm (6985175000000000000♠1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 6986150000000000000♠15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.