atomic number
... to protons and neutrons, electrons are very small. In fact, it would take about 1,800 electrons to equal one proton. The mass of the electron is so small that it is usually thought of as almost zero. The charges of protons and electrons are opposite, but equal, so their charges cancel each other out ...
... to protons and neutrons, electrons are very small. In fact, it would take about 1,800 electrons to equal one proton. The mass of the electron is so small that it is usually thought of as almost zero. The charges of protons and electrons are opposite, but equal, so their charges cancel each other out ...
chap7_nucleus
... the forces that hold the neutrons and protons together. The resulting nucleus therefore has LESS mass than the total mass of the particles before interacting. The Binding Energy is the energy equivalent of the missing mass of a nucleus. Greater binding energy means more energy must be supplied to br ...
... the forces that hold the neutrons and protons together. The resulting nucleus therefore has LESS mass than the total mass of the particles before interacting. The Binding Energy is the energy equivalent of the missing mass of a nucleus. Greater binding energy means more energy must be supplied to br ...
Atomic and Nuclear Terms
... ► Transmutation – Nuclear change of one element into another. • In natural transmutations the nucleus decays spontaneously. There is only one nucleus that undergoes the transformation. • artificial transmutation is induced by the bombardment of the nucleus by high-energy particles. • In order to bal ...
... ► Transmutation – Nuclear change of one element into another. • In natural transmutations the nucleus decays spontaneously. There is only one nucleus that undergoes the transformation. • artificial transmutation is induced by the bombardment of the nucleus by high-energy particles. • In order to bal ...
chemistry i - surrattchemistry
... 3. A biochemist is performing an experiment to determine the effects of Chemical X on the growth of bacteria. Which tube is the experimental control? a. Test tube 1 b. Test tube 2 c. Test tube 3 d. Test tube 4 Objective 2.01: Analyze the historical development of the current atomic theory. 4. Which ...
... 3. A biochemist is performing an experiment to determine the effects of Chemical X on the growth of bacteria. Which tube is the experimental control? a. Test tube 1 b. Test tube 2 c. Test tube 3 d. Test tube 4 Objective 2.01: Analyze the historical development of the current atomic theory. 4. Which ...
Vocabulary Cards
... Atoms of an element that have the same number of protons but different numbers of neutrons, and therefore a different atomic mass. ...
... Atoms of an element that have the same number of protons but different numbers of neutrons, and therefore a different atomic mass. ...
IOSR Journal of Applied Physics (IOSR-JAP)
... general the effect of gravity on electrons can be neglected compared to the electrostatic effects. But the gravitational field becomes important for some astronomical objects like neutrons stars. The gravity and electromagnetic fields manifests themselves as macroscopic potential, while nuclear shor ...
... general the effect of gravity on electrons can be neglected compared to the electrostatic effects. But the gravitational field becomes important for some astronomical objects like neutrons stars. The gravity and electromagnetic fields manifests themselves as macroscopic potential, while nuclear shor ...
Examination 1
... electricity - nuclear power plants Waste - Fission products are highly radioactive themselves, with long half lives. Need to be stored for a long time. No way to speed up or slow down radioactive decay. Nuclear binding energy - energy required to break up a nucleus into its component neutrons and pr ...
... electricity - nuclear power plants Waste - Fission products are highly radioactive themselves, with long half lives. Need to be stored for a long time. No way to speed up or slow down radioactive decay. Nuclear binding energy - energy required to break up a nucleus into its component neutrons and pr ...
Nuclear Chemistry
... electricity - nuclear power plants Waste - Fission products are highly radioactive themselves, with long half lives. Need to be stored for a long time. No way to speed up or slow down radioactive decay. Nuclear binding energy - energy required to break up a nucleus into its component neutrons and pr ...
... electricity - nuclear power plants Waste - Fission products are highly radioactive themselves, with long half lives. Need to be stored for a long time. No way to speed up or slow down radioactive decay. Nuclear binding energy - energy required to break up a nucleus into its component neutrons and pr ...
Elementary my dear Watson review
... When two or more atoms join together, they form molecules. A molecule could be made up of atoms of the same kind. For example: O2 (oxygen) and 03 (ozone). A molecule could also be made up of different atoms. We often call these compounds. Here are some ...
... When two or more atoms join together, they form molecules. A molecule could be made up of atoms of the same kind. For example: O2 (oxygen) and 03 (ozone). A molecule could also be made up of different atoms. We often call these compounds. Here are some ...
CH_8_nucleus_new
... the forces that hold the neutrons and protons together. The resulting nucleus therefore has LESS mass than the total mass of the particles before interacting. The Binding Energy is the energy equivalent of the missing mass of a nucleus. Greater binding energy means more energy must be supplied to br ...
... the forces that hold the neutrons and protons together. The resulting nucleus therefore has LESS mass than the total mass of the particles before interacting. The Binding Energy is the energy equivalent of the missing mass of a nucleus. Greater binding energy means more energy must be supplied to br ...
Radioactive Decay
... • Radiation: rays and particles emitted by radioactive material • Radioactive atoms go through changes that alter their identity – aka changes from one atom to another • How can this happen? ...
... • Radiation: rays and particles emitted by radioactive material • Radioactive atoms go through changes that alter their identity – aka changes from one atom to another • How can this happen? ...
Chapter 1 Section 1
... o The scientists learned that there was something else in an atom that had mass. o That something else had no charge. • Neutron – electrically neutral particle that has the same mass as a proton and is found in an atom’s nucleus. o It took another 20 years for scientists to prove this ...
... o The scientists learned that there was something else in an atom that had mass. o That something else had no charge. • Neutron – electrically neutral particle that has the same mass as a proton and is found in an atom’s nucleus. o It took another 20 years for scientists to prove this ...
Atomic Structure
... The discovery of neutron was actually made about 20 years after the structure of atom was elucidated by Rutherford. Atomic masses of different atoms could not be explained if it was accepted that atoms consisted only of protons and electrons. Thus, Rutherford (1920) suggested that in an atom, there ...
... The discovery of neutron was actually made about 20 years after the structure of atom was elucidated by Rutherford. Atomic masses of different atoms could not be explained if it was accepted that atoms consisted only of protons and electrons. Thus, Rutherford (1920) suggested that in an atom, there ...
Atomic nucleus
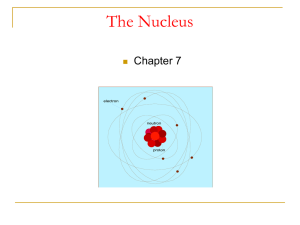
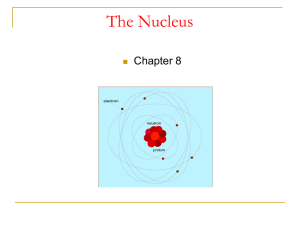
The nucleus is the small, dense region consisting of protons and neutrons at the center of an atom. The atomic nucleus was discovered in 1911 by Ernest Rutherford based on the 1909 Geiger–Marsden gold foil experiment. After the discovery of the neutron in 1932, models for a nucleus composed of protons and neutrons were quickly developed by Dmitri Ivanenko and Werner Heisenberg. Almost all of the mass of an atom is located in the nucleus, with a very small contribution from the electron cloud. Protons and neutrons are bound together to form a nucleus by the nuclear force.The diameter of the nucleus is in the range of 6985175000000000000♠1.75 fm (6985175000000000000♠1.75×10−15 m) for hydrogen (the diameter of a single proton) to about 6986150000000000000♠15 fm for the heaviest atoms, such as uranium. These dimensions are much smaller than the diameter of the atom itself (nucleus + electron cloud), by a factor of about 23,000 (uranium) to about 145,000 (hydrogen).The branch of physics concerned with the study and understanding of the atomic nucleus, including its composition and the forces which bind it together, is called nuclear physics.