4-1 The lowest energy state of an atom is its ground state. (usually
... cause electrons to jump levels. ...
... cause electrons to jump levels. ...
5.3_Matter_Waves
... Everything (photons, electrons, SMU students, planets, ..) has a probability wave - de Broglie Wavelength λ = h = Planck’s constant p momentum ...
... Everything (photons, electrons, SMU students, planets, ..) has a probability wave - de Broglie Wavelength λ = h = Planck’s constant p momentum ...
HW 8
... Which of the following provided evidence that the electrons in atoms are arranged in distinct energy levels? 1. the results of the Millikan oil-drop experiment 2. the existence of elements with noninteger values for atomic weights 3. the scattering of α particles by a metal foil 4. the deflection of ...
... Which of the following provided evidence that the electrons in atoms are arranged in distinct energy levels? 1. the results of the Millikan oil-drop experiment 2. the existence of elements with noninteger values for atomic weights 3. the scattering of α particles by a metal foil 4. the deflection of ...
Atoms, electrons, nuclei J.J. Thomson discovered the electron (1897
... anode is reached only by those electrons that have enough kinetic energy Ek to overcome the work eUanode: Ek ≥ eUanode electron collide with many Mercury atoms, if Ugrid < U*, these collisions will always be elastic: no energy loss and anode current (I) will increase; if Ugrid = U*, collisions might ...
... anode is reached only by those electrons that have enough kinetic energy Ek to overcome the work eUanode: Ek ≥ eUanode electron collide with many Mercury atoms, if Ugrid < U*, these collisions will always be elastic: no energy loss and anode current (I) will increase; if Ugrid = U*, collisions might ...
Review Exam #1 - Seattle Central College
... Characteristics (Electrons have Mass and Momentum) Electromagnetic Radiation (Light) has Both Wave Characteristics (Waves can be Diffracted by Crystals) and Particle Characteristics (Photons have Momentum, but No Mass) Emission spectra of elements demonstrate that the energy of electrons is quantize ...
... Characteristics (Electrons have Mass and Momentum) Electromagnetic Radiation (Light) has Both Wave Characteristics (Waves can be Diffracted by Crystals) and Particle Characteristics (Photons have Momentum, but No Mass) Emission spectra of elements demonstrate that the energy of electrons is quantize ...
Answer on Question #66811, Physics / Electromagnetism An
... Answer on Question #66811, Physics / Electromagnetism An electron moving parallel to the x-axis has an initial speed of 3.70×(10)^6 m/s at the origin. It's speed is reduced to 1.40×(10)^5 m/s at the point x=2c.m -calculate the electric potenial difference between the origin and that point? Find: vE ...
... Answer on Question #66811, Physics / Electromagnetism An electron moving parallel to the x-axis has an initial speed of 3.70×(10)^6 m/s at the origin. It's speed is reduced to 1.40×(10)^5 m/s at the point x=2c.m -calculate the electric potenial difference between the origin and that point? Find: vE ...
Chapter 5: Electrons In Atoms
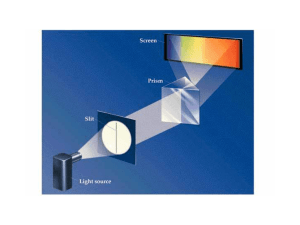
... levels, and these electron lose energy by emitting light when they return to lower energy levels. Ordinary light is a mixture of all wavelengths, but light emitted by ...
... levels, and these electron lose energy by emitting light when they return to lower energy levels. Ordinary light is a mixture of all wavelengths, but light emitted by ...
Quantum Numbers Power Point NOTES
... XX – Group # assigned in blocks to each state XXXX – Serial # assigned in blocks to each state SSN = capacity of nearly 1 billion numbers …as of November 1982 = ~ 277 million number issued leaving 75% still available. ...
... XX – Group # assigned in blocks to each state XXXX – Serial # assigned in blocks to each state SSN = capacity of nearly 1 billion numbers …as of November 1982 = ~ 277 million number issued leaving 75% still available. ...
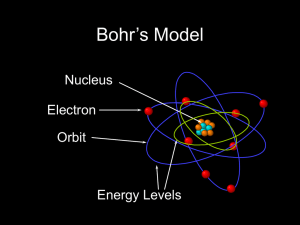
Bohr Atom
... The Bohr model of the atom, like many ideas in the history of science, was at first prompted by and later partially disproved by experimentation. http://en.wikipedia.org/wiki/Category:Chemistry ...
... The Bohr model of the atom, like many ideas in the history of science, was at first prompted by and later partially disproved by experimentation. http://en.wikipedia.org/wiki/Category:Chemistry ...
Atomic Structure Practice Answers
... 5. This is a possible configuration for a transition metal atom. B 6. This electron configuration is not possible. A 7. This is a possible configuration for a transition metal ion. C 8-11 refer to the following: A. B. C. D. E. ...
... 5. This is a possible configuration for a transition metal atom. B 6. This electron configuration is not possible. A 7. This is a possible configuration for a transition metal ion. C 8-11 refer to the following: A. B. C. D. E. ...
Chapter 5 Worksheet 1
... 2. Why does the observation of a very small object such as an electron cause the electron to have its motion changed? The positions of very small objects like electrons can only be studied accurately by hitting them with very high energy (x-rays, gamma rays, etc.) and since the electron is so small ...
... 2. Why does the observation of a very small object such as an electron cause the electron to have its motion changed? The positions of very small objects like electrons can only be studied accurately by hitting them with very high energy (x-rays, gamma rays, etc.) and since the electron is so small ...
Ch 11 WS Orbitals and Electron Arrangement
... 9. Principal energy levels are assigned values in order of ______________________ energy: n = 1, 2, 3, 4, and so forth. 10. In the quantum mechanical model the regions where electrons are likely to be found are called ______________________ and are denoted by______________________ . ...
... 9. Principal energy levels are assigned values in order of ______________________ energy: n = 1, 2, 3, 4, and so forth. 10. In the quantum mechanical model the regions where electrons are likely to be found are called ______________________ and are denoted by______________________ . ...
Section 4-2 The Quantum Model of the Atom Problems with the Bohr
... c. As “n” increases, the electron's energy and distance from the nucleus increase d. Multiple electrons can share a value of “n”, this is called a “shell” of electrons e. Total possible number of orbitals in any value of “n” = n2. II. Angular Momentum Quantum Number: “L” a. “L” differentiates sublev ...
... c. As “n” increases, the electron's energy and distance from the nucleus increase d. Multiple electrons can share a value of “n”, this is called a “shell” of electrons e. Total possible number of orbitals in any value of “n” = n2. II. Angular Momentum Quantum Number: “L” a. “L” differentiates sublev ...
No Slide Title
... emission spectrum that did not match known emission lines Mystery element was named Helium In 1895, William Ramsey discovered helium in a mineral of uranium (from alpha decay). ...
... emission spectrum that did not match known emission lines Mystery element was named Helium In 1895, William Ramsey discovered helium in a mineral of uranium (from alpha decay). ...
chapter-27-1-with
... Max Planck found he could explain these curves if he assumed that electromagnetic energy was radiated in ...
... Max Planck found he could explain these curves if he assumed that electromagnetic energy was radiated in ...
Bohr Model of the Hydrogen Atom
... still a pleasing result which can be obtained without fancy electromagnetic techniques. One of the things which ultimately led physicists to suspect that this picture was completely wrong is the fact that accelerating charges radiate: i.e., they lose energy to electromagnetic radiation. The rate at ...
... still a pleasing result which can be obtained without fancy electromagnetic techniques. One of the things which ultimately led physicists to suspect that this picture was completely wrong is the fact that accelerating charges radiate: i.e., they lose energy to electromagnetic radiation. The rate at ...
Section 25.2 Name_____________________
... 3. Use the following word bank to complete the section below: atomic number nuclear bombardment ...
... 3. Use the following word bank to complete the section below: atomic number nuclear bombardment ...
REVIEW and REINFORCEMENT Structure of the Atom
... Use your knowledge of atomic number and mass number to fill in the missing numbers: HOW MANY? Element ...
... Use your knowledge of atomic number and mass number to fill in the missing numbers: HOW MANY? Element ...
Electron

The electron is a subatomic particle, symbol e− or β−, with a negative elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value in units of ħ, which means that it is a fermion. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all matter, electrons have properties of both particles and waves, and so can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a higher De Broglie wavelength for typical energies.Many physical phenomena involve electrons in an essential role, such as electricity, magnetism, and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. An electron generates an electric field surrounding it. An electron moving relative to an observer generates a magnetic field. External magnetic fields deflect an electron. Electrons radiate or absorb energy in the form of photons when accelerated. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma using electromagnetic fields, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.Interactions involving electrons and other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between positive protons inside atomic nuclei and negative electrons composes atoms. Ionization or changes in the proportions of particles changes the binding energy of the system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms in 1838; Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.