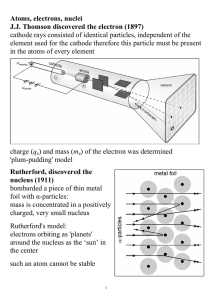
Atoms, electrons, nuclei J.J. Thomson discovered the electron (1897
... anode is reached only by those electrons that have enough kinetic energy Ek to overcome the work eUanode: Ek ≥ eUanode electron collide with many Mercury atoms, if Ugrid < U*, these collisions will always be elastic: no energy loss and anode current (I) will increase; if Ugrid = U*, collisions might ...
... anode is reached only by those electrons that have enough kinetic energy Ek to overcome the work eUanode: Ek ≥ eUanode electron collide with many Mercury atoms, if Ugrid < U*, these collisions will always be elastic: no energy loss and anode current (I) will increase; if Ugrid = U*, collisions might ...
19.1 Reinforcement WKT to project
... 1. How is the chemical symbol of an element determined? 2. Of what are atoms composed? 3. Are electrons, protons, or neutrons the smallest particles? If not, what are? 4. How many types of quarks are there and what is the name of one of them? 5. Why do scientists use models to study atoms? 6. Why ha ...
... 1. How is the chemical symbol of an element determined? 2. Of what are atoms composed? 3. Are electrons, protons, or neutrons the smallest particles? If not, what are? 4. How many types of quarks are there and what is the name of one of them? 5. Why do scientists use models to study atoms? 6. Why ha ...
Seeing Atoms and Electrons in Motion - The Munich
... (b) Compressing single-electron wavepackets with laser fields for reaching attosecond+picometer resolution in electron diffraction. (c) Electron microscopy/spectroscopy of electromagnetic resonances and quantum interferences at plasmonic subwavelength structures. Our research is located at the Max-P ...
... (b) Compressing single-electron wavepackets with laser fields for reaching attosecond+picometer resolution in electron diffraction. (c) Electron microscopy/spectroscopy of electromagnetic resonances and quantum interferences at plasmonic subwavelength structures. Our research is located at the Max-P ...
Cathode ray deflection tube
... The electron gun shoots out a beam of electrons across an evacuated tube. It hits a fluorescent screen placed in its path and when it does the screen glows. If there is no voltage between the two plates the beam will go along the middle of the scale. Beams of electrons (cathode rays) move in straigh ...
... The electron gun shoots out a beam of electrons across an evacuated tube. It hits a fluorescent screen placed in its path and when it does the screen glows. If there is no voltage between the two plates the beam will go along the middle of the scale. Beams of electrons (cathode rays) move in straigh ...
Louie de Broglie
... The Heisenberg Uncertainty Principle Heisenberg concluded that it is impossible to make any measurement on an object without disturbing it – at least a little. Electrons are detected by photons and because a photon and an electron have the same energy, any attempt to locate an electron with a pho ...
... The Heisenberg Uncertainty Principle Heisenberg concluded that it is impossible to make any measurement on an object without disturbing it – at least a little. Electrons are detected by photons and because a photon and an electron have the same energy, any attempt to locate an electron with a pho ...
IPS Unit 8 – Periodic Table Structure of the Atom Worksheet
... 1. How is the chemical symbol of an element written? ...
... 1. How is the chemical symbol of an element written? ...
Electronic Structure and the Periodic Table A. Bohr Model of the
... A. Bohr Model of the Atom 1. Solar System Model 2. Created to Fit a “Quantized” Picture of Energy Transfer 3. Basis: Noncontinuous Emission Spectra of the Elements 4. Basic Postulates a. Electrons reside in certain allowed energy states b. Energy absorption and emission by atoms is caused by electro ...
... A. Bohr Model of the Atom 1. Solar System Model 2. Created to Fit a “Quantized” Picture of Energy Transfer 3. Basis: Noncontinuous Emission Spectra of the Elements 4. Basic Postulates a. Electrons reside in certain allowed energy states b. Energy absorption and emission by atoms is caused by electro ...
FREE ELECTRON THEORY
... crystal. A particle-like entity can be obtained by superposing the plane wave states to form a wavepacket. ...
... crystal. A particle-like entity can be obtained by superposing the plane wave states to form a wavepacket. ...
Math Module II Review
... This is an awesome light show caused by the atoms of the atmosphere getting their electrons “excited” by high energy particles from the sun. It happens near the poles due to the magnetic field of the Earth being thinner there. ...
... This is an awesome light show caused by the atoms of the atmosphere getting their electrons “excited” by high energy particles from the sun. It happens near the poles due to the magnetic field of the Earth being thinner there. ...
CH1710 HW#7 (2017)-Quanta, electron config
... 1. An FM station broadcasts classical music at 93.5 MHz (megahertz or 106 Hz). Find the wavelength (in m, nm and Å) of these radio waves. ...
... 1. An FM station broadcasts classical music at 93.5 MHz (megahertz or 106 Hz). Find the wavelength (in m, nm and Å) of these radio waves. ...
The Quantum Atom (section 18)
... 5P revision notes: Section 18: The Quantum Atom JJ Thomson 1899 – discovered electron using cathode ray tube (diagrams p210 Adams& Allday, p784 Muncaster). Particles given off from cathode always have the same charge to mass ratio, no matter what element the cathode is made of. Conclusion – they are ...
... 5P revision notes: Section 18: The Quantum Atom JJ Thomson 1899 – discovered electron using cathode ray tube (diagrams p210 Adams& Allday, p784 Muncaster). Particles given off from cathode always have the same charge to mass ratio, no matter what element the cathode is made of. Conclusion – they are ...
3. THE DEGENERATE ELECTRON GAS Chapter 1 : SECOND QUANTIZATION
... Chapter 1 : SECOND QUANTIZATION ...
... Chapter 1 : SECOND QUANTIZATION ...
Quantum_PPT
... • A change in a electric field produced a magnetic field • A change in a magnetic field produced an electric field ...
... • A change in a electric field produced a magnetic field • A change in a magnetic field produced an electric field ...
Wave Particle Duality File
... both real. • We need both ideas to explain the behaviour of light. • This is called wave-particle duality ...
... both real. • We need both ideas to explain the behaviour of light. • This is called wave-particle duality ...
Atomic-Structure-Concise-Notes
... Electronic Structure of an Atom 5. Electronic structure of atom: - Shell closest to nucleus will have lowest energy. - Principle quantum shell: n=1, 2, 3, … - Sub-shell: s, p, d, and f - Orbital: s, px, py, pz, dxy, dxz, dyz, dx2-y2, dz2 - In a nutshell, atoms consists of various principle quantum s ...
... Electronic Structure of an Atom 5. Electronic structure of atom: - Shell closest to nucleus will have lowest energy. - Principle quantum shell: n=1, 2, 3, … - Sub-shell: s, p, d, and f - Orbital: s, px, py, pz, dxy, dxz, dyz, dx2-y2, dz2 - In a nutshell, atoms consists of various principle quantum s ...
Constructive Interference
... exist in stable configurations around nuclei Wavefunctions and energies for these configurations determine most properties of matter ...
... exist in stable configurations around nuclei Wavefunctions and energies for these configurations determine most properties of matter ...
Classical theory of atomic structure
... theory fails to explain the stability of atomic structure as accelerated electron radiates energy in the form of electromagnetic waves and consequently it will loose energy gradually and at last fall into the nucleus due to gravitational and coulombic attractions. Secondly classical theory fails to ...
... theory fails to explain the stability of atomic structure as accelerated electron radiates energy in the form of electromagnetic waves and consequently it will loose energy gradually and at last fall into the nucleus due to gravitational and coulombic attractions. Secondly classical theory fails to ...
Electron

The electron is a subatomic particle, symbol e− or β−, with a negative elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value in units of ħ, which means that it is a fermion. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all matter, electrons have properties of both particles and waves, and so can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a higher De Broglie wavelength for typical energies.Many physical phenomena involve electrons in an essential role, such as electricity, magnetism, and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. An electron generates an electric field surrounding it. An electron moving relative to an observer generates a magnetic field. External magnetic fields deflect an electron. Electrons radiate or absorb energy in the form of photons when accelerated. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma using electromagnetic fields, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.Interactions involving electrons and other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between positive protons inside atomic nuclei and negative electrons composes atoms. Ionization or changes in the proportions of particles changes the binding energy of the system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms in 1838; Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.