Chemistry TEST 4 Review and Answers
... Page 165 – 171 and 197 – 203 in textbook 5.1 Wave and Particle characteristics of Light ...
... Page 165 – 171 and 197 – 203 in textbook 5.1 Wave and Particle characteristics of Light ...
Quantum Numbers Practice Problems Name: AP Physics Period: 1
... 2. According to the deBroglie theory, electrons orbiting around the nucleus of an atom are waves (as well as particles). How does our understanding of electron waves change when we give up the idea of a simple orbit for an electron (like the ones that Bohr envisioned)? ...
... 2. According to the deBroglie theory, electrons orbiting around the nucleus of an atom are waves (as well as particles). How does our understanding of electron waves change when we give up the idea of a simple orbit for an electron (like the ones that Bohr envisioned)? ...
Electron Configuration
... To find the electron configuration of a given element, simply locate its position on the periodic table ...
... To find the electron configuration of a given element, simply locate its position on the periodic table ...
History of the Atom
... Based on earlier work by E. Goldstein (1886) Millikan, Thomson and coworkers proposed the presence of a positively charged particle called the proton Goldstein observed what he called canal rays while using a cathode ray tube with the rays traveling in the opposite direction of Thomson’s ...
... Based on earlier work by E. Goldstein (1886) Millikan, Thomson and coworkers proposed the presence of a positively charged particle called the proton Goldstein observed what he called canal rays while using a cathode ray tube with the rays traveling in the opposite direction of Thomson’s ...
Quantum
... A nuclear reaction is when the particles of an atom change. All elements that are larger than Bismuth (with some exceptions) have radioactivity and will decay into smaller elements. These reactions can result into different isotopes and even different atoms when these reactions occur. Nuclear reacti ...
... A nuclear reaction is when the particles of an atom change. All elements that are larger than Bismuth (with some exceptions) have radioactivity and will decay into smaller elements. These reactions can result into different isotopes and even different atoms when these reactions occur. Nuclear reacti ...
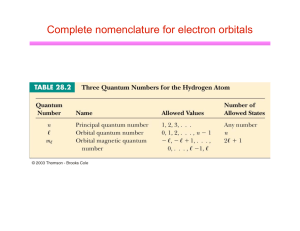
Complete nomenclature for electron orbitals
... periodic table towards higher Z, electrons fill in each subshell starting from the lowest energy level l Each subshell has 2(2l+1) electrons in it l H (1 electron) is described by either of quantum numbers (1,0,0,1/2) or (1,0,0,-1/2) u 1s1 l He (2 electrons) is described by quantum numbers (1,0,0,1/ ...
... periodic table towards higher Z, electrons fill in each subshell starting from the lowest energy level l Each subshell has 2(2l+1) electrons in it l H (1 electron) is described by either of quantum numbers (1,0,0,1/2) or (1,0,0,-1/2) u 1s1 l He (2 electrons) is described by quantum numbers (1,0,0,1/ ...
The Drude Model An intuitive picture of electron motion
... This model shows us that electric fields cause charges to move (electric current) by the Lorentz force, and electron scattering causes resistance. In Module 2 there is a discussion about whether ‘elect ...
... This model shows us that electric fields cause charges to move (electric current) by the Lorentz force, and electron scattering causes resistance. In Module 2 there is a discussion about whether ‘elect ...
Problem Set 05
... c) An accelerating charge will lose energy due to radiation. Show that this will lead to a decrease in the electron orbital radius described by ...
... c) An accelerating charge will lose energy due to radiation. Show that this will lead to a decrease in the electron orbital radius described by ...
AP Chem II Instructor: Mr. Malasky Name Period ______ Due Date
... ____ 1. The Heisenberg uncertainty principle states that a. electrons have no momentum b. the position of an electron is impossible to determine c. the faster an electron moves, the more unreliable is its energy d. the momentum and the position of an electron cannot be precisely defined simultaneous ...
... ____ 1. The Heisenberg uncertainty principle states that a. electrons have no momentum b. the position of an electron is impossible to determine c. the faster an electron moves, the more unreliable is its energy d. the momentum and the position of an electron cannot be precisely defined simultaneous ...
Chapter 4 Exam Review Democritus named tiny pieces of matter
... 19. What do scientists use to predict the locations of electrons in atoms? _______________________________ 20. What does the electron cloud model describe? _________________________________________________________ 21. How many electrons can one orbital contain? ________________________ 22. An electr ...
... 19. What do scientists use to predict the locations of electrons in atoms? _______________________________ 20. What does the electron cloud model describe? _________________________________________________________ 21. How many electrons can one orbital contain? ________________________ 22. An electr ...
vocab chap 6
... packed nucleus and that atoms are mostly empty space; also discovered the proton ...
... packed nucleus and that atoms are mostly empty space; also discovered the proton ...
Unit 2 Review KEY
... Frequency (v) – number of waves that pass a given point in a specific time (1 sec) Photoelectric Effect – an emission of electrons from a metal when light shines on a metal. Quantum – minimum quantity of energy that can be lost or gained by an atom. Photon – particle of electromagnetic radiation hav ...
... Frequency (v) – number of waves that pass a given point in a specific time (1 sec) Photoelectric Effect – an emission of electrons from a metal when light shines on a metal. Quantum – minimum quantity of energy that can be lost or gained by an atom. Photon – particle of electromagnetic radiation hav ...
Atomic Orbitals - Daytona State College
... An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also r ...
... An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also r ...
Electron

The electron is a subatomic particle, symbol e− or β−, with a negative elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value in units of ħ, which means that it is a fermion. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all matter, electrons have properties of both particles and waves, and so can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a higher De Broglie wavelength for typical energies.Many physical phenomena involve electrons in an essential role, such as electricity, magnetism, and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. An electron generates an electric field surrounding it. An electron moving relative to an observer generates a magnetic field. External magnetic fields deflect an electron. Electrons radiate or absorb energy in the form of photons when accelerated. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma using electromagnetic fields, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.Interactions involving electrons and other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between positive protons inside atomic nuclei and negative electrons composes atoms. Ionization or changes in the proportions of particles changes the binding energy of the system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms in 1838; Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.