Why do elements combine
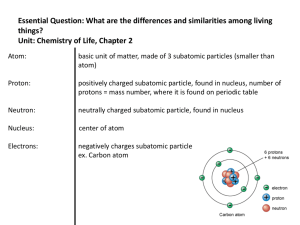
... Ion- an electrically charged particle formed by the transfer of electrons (+ or - charge) The charge of an ion is related to its valence number ⇒ Elements in Groups 1A, 2A, 3A form positive ions (+1, ...
... Ion- an electrically charged particle formed by the transfer of electrons (+ or - charge) The charge of an ion is related to its valence number ⇒ Elements in Groups 1A, 2A, 3A form positive ions (+1, ...
Name
... same as the emission spectrum of another element. 16. Only electrons moving from energy levels lose energy and emit light. ...
... same as the emission spectrum of another element. 16. Only electrons moving from energy levels lose energy and emit light. ...
Definitions are in Book
... False. Einstein helped lay some ground work that was eventually used to prove quantum mechanics, but he rejected the idea that energy was quantized (which is the heart and soul of quantum mechanics). 5) In really high energy states, the electrons in some radioactive elements occasionally enter an or ...
... False. Einstein helped lay some ground work that was eventually used to prove quantum mechanics, but he rejected the idea that energy was quantized (which is the heart and soul of quantum mechanics). 5) In really high energy states, the electrons in some radioactive elements occasionally enter an or ...
qp2
... of light (energy packet), thus showing that light behaves as both particle and wave. The next riddle related to the property of electrons and Bohr's model of an atom. The laws of classical physics stated that an accelerated charge would give off electromagnetic waves, thereby losing energy all the t ...
... of light (energy packet), thus showing that light behaves as both particle and wave. The next riddle related to the property of electrons and Bohr's model of an atom. The laws of classical physics stated that an accelerated charge would give off electromagnetic waves, thereby losing energy all the t ...
Electrostatic attraction between oppositely charged ions. A shared
... Electrostatic attraction between oppositely charged ions. A shared pair of electrons between two nuclei, where the electrons are attracted to each nucleus. A shared pair of electrons between two atoms where one atom contributes both electrons to the bond. The electrostatic attraction between a regul ...
... Electrostatic attraction between oppositely charged ions. A shared pair of electrons between two nuclei, where the electrons are attracted to each nucleus. A shared pair of electrons between two atoms where one atom contributes both electrons to the bond. The electrostatic attraction between a regul ...
Poster - high school teachers at CERN
... Introduction Electrons have both wave and particle behaviours that will show up depending on the type of the experiments, i.e., experiments measuring particle properties will not give the wave behaviour and vice versa. According to de Broglie equation, fast electrons have high momentum and hence a w ...
... Introduction Electrons have both wave and particle behaviours that will show up depending on the type of the experiments, i.e., experiments measuring particle properties will not give the wave behaviour and vice versa. According to de Broglie equation, fast electrons have high momentum and hence a w ...
The Hydrogen Atom
... The light source alone would give a continuous spectrum, but atoms of the gas absorb certain frequencies from the light. The lines in the emission and absorption spectrum of the same chemical element have the same frequencies. Frequencies in the spectrum of an element fall into sets called spectral ...
... The light source alone would give a continuous spectrum, but atoms of the gas absorb certain frequencies from the light. The lines in the emission and absorption spectrum of the same chemical element have the same frequencies. Frequencies in the spectrum of an element fall into sets called spectral ...
Quantum Numbers and Electron Configurations Worksheet
... Use a phrase to describe why the 2s orbital is more stable (lower energy) versus 2p. When you superimpose the total radial probability of 2s and 2p onto the plot of 1s, you notice that the 2s has a small peak that is inside the 1s shield, which causes them to have more exposure to the full nuclear c ...
... Use a phrase to describe why the 2s orbital is more stable (lower energy) versus 2p. When you superimpose the total radial probability of 2s and 2p onto the plot of 1s, you notice that the 2s has a small peak that is inside the 1s shield, which causes them to have more exposure to the full nuclear c ...
14. Elementary Particles
... Einstein had defined the photon in 1905. The proton is the nucleus of the hydrogen atom (let’s give Rutherford credit for its discovery). In 1932 James Chadwick identified the neutron, actually first seen by Bothe and Becker. That seemed sufficient… ...
... Einstein had defined the photon in 1905. The proton is the nucleus of the hydrogen atom (let’s give Rutherford credit for its discovery). In 1932 James Chadwick identified the neutron, actually first seen by Bothe and Becker. That seemed sufficient… ...
Resistance of constantan wire
... As you may know the current in a wire is due to the motion of free electrons within the wire. These electrons are not bound to any particular atom but are free to 'wander' through the body of the material. The more free electrons per unit volume the greater the current for a given voltage difference ...
... As you may know the current in a wire is due to the motion of free electrons within the wire. These electrons are not bound to any particular atom but are free to 'wander' through the body of the material. The more free electrons per unit volume the greater the current for a given voltage difference ...
Practice Exam III
... 1. Which of the following is a statement of Hess's law? A) If a reaction is carried out in a series of steps, the ΔH for the reaction will equal the sum of the enthalpy changes for the individual steps. B) If a reaction is carried out in a series of steps, the ΔH for the reaction will equal the prod ...
... 1. Which of the following is a statement of Hess's law? A) If a reaction is carried out in a series of steps, the ΔH for the reaction will equal the sum of the enthalpy changes for the individual steps. B) If a reaction is carried out in a series of steps, the ΔH for the reaction will equal the prod ...
Physics 200 Class #1 Outline
... bigger than the electron. For the electron accelerated with 10,000 Volts, moving pretty fast, the wavelength is 0.01 nm, or 1 x 10 -11 m. For a neutron moving the same speed, the wavelength would be 0.0002 nm or 2 x 10 -13 m. That's a million times smaller wavelength than visible light! If light beh ...
... bigger than the electron. For the electron accelerated with 10,000 Volts, moving pretty fast, the wavelength is 0.01 nm, or 1 x 10 -11 m. For a neutron moving the same speed, the wavelength would be 0.0002 nm or 2 x 10 -13 m. That's a million times smaller wavelength than visible light! If light beh ...
ATOMIC PHYSICS
... explain, qualitatively, the concept of stationary states and how they explain the observed spectra of atoms and molecules calculate the energy difference between states, using the law of conservation of energy and the observed characteristics of an emitted photon Questions: Use the following informa ...
... explain, qualitatively, the concept of stationary states and how they explain the observed spectra of atoms and molecules calculate the energy difference between states, using the law of conservation of energy and the observed characteristics of an emitted photon Questions: Use the following informa ...
Answers - Manhattan Press
... Cathode rays can travel in vacuum (vacuum tube), which is a distinctive property of electromagnetic wave. ...
... Cathode rays can travel in vacuum (vacuum tube), which is a distinctive property of electromagnetic wave. ...
polarization of the allotropic hollow foms of carbon and its use in
... The analytical model of polarizing resonant interactions hollow forms of carbon with the quantum charged particles with total energy E > 0 is offered. The problem is shown to classical quantum-mechanical effect: «a particle in a box» (a Q-particle) in which power conditions are defined by the sizes ...
... The analytical model of polarizing resonant interactions hollow forms of carbon with the quantum charged particles with total energy E > 0 is offered. The problem is shown to classical quantum-mechanical effect: «a particle in a box» (a Q-particle) in which power conditions are defined by the sizes ...
Electron

The electron is a subatomic particle, symbol e− or β−, with a negative elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value in units of ħ, which means that it is a fermion. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all matter, electrons have properties of both particles and waves, and so can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a higher De Broglie wavelength for typical energies.Many physical phenomena involve electrons in an essential role, such as electricity, magnetism, and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. An electron generates an electric field surrounding it. An electron moving relative to an observer generates a magnetic field. External magnetic fields deflect an electron. Electrons radiate or absorb energy in the form of photons when accelerated. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma using electromagnetic fields, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.Interactions involving electrons and other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between positive protons inside atomic nuclei and negative electrons composes atoms. Ionization or changes in the proportions of particles changes the binding energy of the system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms in 1838; Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.