Rutherford`s Gold Foil Experiment
... Millikan was able to determine the exact charge of an electron by suspending oil droplets in an electric field ...
... Millikan was able to determine the exact charge of an electron by suspending oil droplets in an electric field ...
Structure of Matter Vocab Structure of Matter vocab
... Atoms of the same element that have different numbers of neutrons Example: Boron – 10 and Boron 11 ...
... Atoms of the same element that have different numbers of neutrons Example: Boron – 10 and Boron 11 ...
PHYS 203 General Physics
... 5. What is the Heisenberg Uncertainty Principle? What is there about it that Einstein did not like? 6. Know the contributions of each of the following to our understanding of the structure of the atom, or to our understanding of matter in general: Planck, de Broglie, Rutherford, Bohr, Einstein, and ...
... 5. What is the Heisenberg Uncertainty Principle? What is there about it that Einstein did not like? 6. Know the contributions of each of the following to our understanding of the structure of the atom, or to our understanding of matter in general: Planck, de Broglie, Rutherford, Bohr, Einstein, and ...
Chapter 14
... This is the process by which one element changes into another element. Atoms of the same element with a different number of neutrons are called _. The atomic number of an element is equal to _____________. This is the smallest of the three atomic particles. The positive electrode in a CRT is called ...
... This is the process by which one element changes into another element. Atoms of the same element with a different number of neutrons are called _. The atomic number of an element is equal to _____________. This is the smallest of the three atomic particles. The positive electrode in a CRT is called ...
Developing a new physics for atoms
... the e- will be found at that location: probability density or electron density. This concept of electron density plots led to the development of electron orbitals. ...
... the e- will be found at that location: probability density or electron density. This concept of electron density plots led to the development of electron orbitals. ...
Light Emitting Diodes
... When the negative end of the circuit is hooked up to the N-type layer and the positive end is hooked up to P-type layer, electrons and holes start moving and the depletion zone disappears. ...
... When the negative end of the circuit is hooked up to the N-type layer and the positive end is hooked up to P-type layer, electrons and holes start moving and the depletion zone disappears. ...
Chapter 4: Struct of Atom
... S Therefore, in a 6 x 10^-7 m thick foil one would have ~ 6 x 10^-7 div. by 2.6 x 10^-10 ~ 2,300 atoms. So, one expected that the alpha S => <θ> = √2300 * 0.016 ~ 0.8° ...
... S Therefore, in a 6 x 10^-7 m thick foil one would have ~ 6 x 10^-7 div. by 2.6 x 10^-10 ~ 2,300 atoms. So, one expected that the alpha S => <θ> = √2300 * 0.016 ~ 0.8° ...
PPTX
... • Note that dE/dx depends on bg • The same energy loss in gas (or liquid gas, e.g. in a bubble chamber) for 10 GeV muon and 100 GeV proton ...
... • Note that dE/dx depends on bg • The same energy loss in gas (or liquid gas, e.g. in a bubble chamber) for 10 GeV muon and 100 GeV proton ...
03 Homework File
... To be handed in: Tuesday 31st January Answer scripts should be returned in the box provided on the first floor in the Physics Building (G.O. Jones) by 4pm on the day quoted above. Course title, week number and student’s name should appear on every sheet of the worked exercises, which should be secur ...
... To be handed in: Tuesday 31st January Answer scripts should be returned in the box provided on the first floor in the Physics Building (G.O. Jones) by 4pm on the day quoted above. Course title, week number and student’s name should appear on every sheet of the worked exercises, which should be secur ...
Electron Configurations
... So you learned about the Bohr model of an atom as well the electronic configuration of that atom. If you have taken or are taking any sort of an advanced chemistry class, then you probably didn’t have much trouble with these concepts. Otherwise, you may want some extra information on the subject. Mo ...
... So you learned about the Bohr model of an atom as well the electronic configuration of that atom. If you have taken or are taking any sort of an advanced chemistry class, then you probably didn’t have much trouble with these concepts. Otherwise, you may want some extra information on the subject. Mo ...
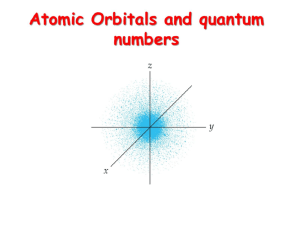
Atomic Orbitals and quantum numbers
... •Therefore, on any given energy level, there can be up to 1s orbital, 3p orbitals, 5d orbitals, and 7f orbitals. ...
... •Therefore, on any given energy level, there can be up to 1s orbital, 3p orbitals, 5d orbitals, and 7f orbitals. ...
N - University of St Andrews
... Pauli exclusion principle: No two electrons have the same set of quantum numbers. This means that we have to occupy several orbitals. Electron configuration: state of occupation of the energy levels (or terms) of an atom by electrons For example, the electron configuration for the ground state of Li ...
... Pauli exclusion principle: No two electrons have the same set of quantum numbers. This means that we have to occupy several orbitals. Electron configuration: state of occupation of the energy levels (or terms) of an atom by electrons For example, the electron configuration for the ground state of Li ...
Exam #2
... (a) Electron affinities decrease going down the group (from smaller to larger elements). (b) Ionization energies decrease going down the group (from smaller to larger elements). (c) Chemical reactivity decreases going down the group (from smaller to larger elements). (d) The second ionization energy ...
... (a) Electron affinities decrease going down the group (from smaller to larger elements). (b) Ionization energies decrease going down the group (from smaller to larger elements). (c) Chemical reactivity decreases going down the group (from smaller to larger elements). (d) The second ionization energy ...
Two-electron Interference
... quantum interference of two independent, but indistinquishable, particles is also possible. For a single particle, the interference is between the amplitudes of the particle’s wave function, whereas the interference between two particles is a direct result of quantum exchange statistics. Such int ...
... quantum interference of two independent, but indistinquishable, particles is also possible. For a single particle, the interference is between the amplitudes of the particle’s wave function, whereas the interference between two particles is a direct result of quantum exchange statistics. Such int ...
Electrons in the Atom
... 3. What is the energy released when a hydrogen electron moves from n=6 to n=2? 4. What is the difference between ground state and excited state? How do electrons move between these two states? 5. What does it mean for an atom to become an ion? How does the charge relate to the change in electrons? ...
... 3. What is the energy released when a hydrogen electron moves from n=6 to n=2? 4. What is the difference between ground state and excited state? How do electrons move between these two states? 5. What does it mean for an atom to become an ion? How does the charge relate to the change in electrons? ...
3. atomic structure
... nucleus where an electron is most likely to be found (probability of location) The exact path of an electron in this area is not known ...
... nucleus where an electron is most likely to be found (probability of location) The exact path of an electron in this area is not known ...
Matter Unit
... mass which is unique to that element. Atoms cannot be subdivided, created, or destroyed in ordinary chemical reactions. However, these changes CAN occur in nuclear reactions! All matter is composed of atoms Atoms of any one element differ in properties from atoms of another element ...
... mass which is unique to that element. Atoms cannot be subdivided, created, or destroyed in ordinary chemical reactions. However, these changes CAN occur in nuclear reactions! All matter is composed of atoms Atoms of any one element differ in properties from atoms of another element ...
Modern Atomic Theory Notes
... The modern model of the atom takes into account both the _____________________ and _____________________ properties of electrons. According to this model, electrons are located in ___________________, regions around a nucleus that correspond to specific energy levels. ...
... The modern model of the atom takes into account both the _____________________ and _____________________ properties of electrons. According to this model, electrons are located in ___________________, regions around a nucleus that correspond to specific energy levels. ...
Electron

The electron is a subatomic particle, symbol e− or β−, with a negative elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value in units of ħ, which means that it is a fermion. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all matter, electrons have properties of both particles and waves, and so can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a higher De Broglie wavelength for typical energies.Many physical phenomena involve electrons in an essential role, such as electricity, magnetism, and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. An electron generates an electric field surrounding it. An electron moving relative to an observer generates a magnetic field. External magnetic fields deflect an electron. Electrons radiate or absorb energy in the form of photons when accelerated. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma using electromagnetic fields, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.Interactions involving electrons and other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between positive protons inside atomic nuclei and negative electrons composes atoms. Ionization or changes in the proportions of particles changes the binding energy of the system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms in 1838; Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.