RAD 107 HOMEWORK 4
... 5. How much kinetic energy must an electron have in order to knock a Tungsten K-shell electron out a Tungsten atom? How much to knock out a Tungsten N-shell electron? [Hint: read the first table in Problem 4.] 6. Suppose that a K-shell electron has been knocked out of a Tungsten atom due to a collis ...
... 5. How much kinetic energy must an electron have in order to knock a Tungsten K-shell electron out a Tungsten atom? How much to knock out a Tungsten N-shell electron? [Hint: read the first table in Problem 4.] 6. Suppose that a K-shell electron has been knocked out of a Tungsten atom due to a collis ...
Alpha particle – a positively charged atom that is released in the
... 12. Nuclear radiation – the particles that are released from the nucleus during Radioactive decay, such as neutrons, electrons, and photons 13. Nucleus – an atom’s central region, which is made up of protons and neutrons 14. Orbital – a region in an atom where there is a high probability of finding ...
... 12. Nuclear radiation – the particles that are released from the nucleus during Radioactive decay, such as neutrons, electrons, and photons 13. Nucleus – an atom’s central region, which is made up of protons and neutrons 14. Orbital – a region in an atom where there is a high probability of finding ...
Carrier Mobility
... • Nearly free electrons can easily move in a semiconductor since they are not part of a chemical bond between atoms. • Valence electrons are shared between atoms. It turns out that a valence electron can also exchange places with another valence electron that is being shared with a different atom. ...
... • Nearly free electrons can easily move in a semiconductor since they are not part of a chemical bond between atoms. • Valence electrons are shared between atoms. It turns out that a valence electron can also exchange places with another valence electron that is being shared with a different atom. ...
The topic that fascinated me the most in my Science lessons this
... Table shows trends and patterns as we move horizontally across a Period and vertically across a Group. The trends and patterns are captured in this version of the Periodic Table obtained from the Internet: ...
... Table shows trends and patterns as we move horizontally across a Period and vertically across a Group. The trends and patterns are captured in this version of the Periodic Table obtained from the Internet: ...
Atomic Structure Guided Notes- Key 1. The simplest form of matter is
... 3. Atomic Number a. The atomic number is equal to the number of protons in the nucleus of an element b. The atomic number identifies the element i. Oxygen Atomic Number= 8, which means there are 8 protons ii. Argon Atomic Number = 18, how many protons? 18 4. Electrons a. Electron Characteristics i. ...
... 3. Atomic Number a. The atomic number is equal to the number of protons in the nucleus of an element b. The atomic number identifies the element i. Oxygen Atomic Number= 8, which means there are 8 protons ii. Argon Atomic Number = 18, how many protons? 18 4. Electrons a. Electron Characteristics i. ...
Questions
... solved exactly. Much of the middle part of the course will be devoted to solving this. One coarse approximation is the “mean-field” approximation, whereby every electron moves in the average field of all the other particles. The mean field is spherically symmetric, and thus, the quantum numbers n an ...
... solved exactly. Much of the middle part of the course will be devoted to solving this. One coarse approximation is the “mean-field” approximation, whereby every electron moves in the average field of all the other particles. The mean field is spherically symmetric, and thus, the quantum numbers n an ...
Document
... It would seem that the basic idea of the quantum theory is the impossibility of imagining an isolated quantity of energy without associating with it a certain frequency de Broglie in 1923 as a graduate student ...
... It would seem that the basic idea of the quantum theory is the impossibility of imagining an isolated quantity of energy without associating with it a certain frequency de Broglie in 1923 as a graduate student ...
Chapter 7 The Quantum- Mechanical Model of the
... Angular Momentum Quantum Number, l subshell, (s,p,d,f blocks of periodic table) • The angular momentum quantum number determines the shape of the orbital. • l can have integer values from 0 to (n – 1). • Each value of l is called by a particular letter that designates the shape of the orbital. – s ...
... Angular Momentum Quantum Number, l subshell, (s,p,d,f blocks of periodic table) • The angular momentum quantum number determines the shape of the orbital. • l can have integer values from 0 to (n – 1). • Each value of l is called by a particular letter that designates the shape of the orbital. – s ...
2008 midtermkey - University of Victoria
... of the atom is INCORRECT? A) Atomic orbitals describe regions in which an electron is most likely to be found around a nucleus. B) The three electrons in the configuration 2p3 have parallel spins (i.e. the same ms value). C) The fact that two electrons in the same atom cannot have the same set of fo ...
... of the atom is INCORRECT? A) Atomic orbitals describe regions in which an electron is most likely to be found around a nucleus. B) The three electrons in the configuration 2p3 have parallel spins (i.e. the same ms value). C) The fact that two electrons in the same atom cannot have the same set of fo ...
PowerPoint
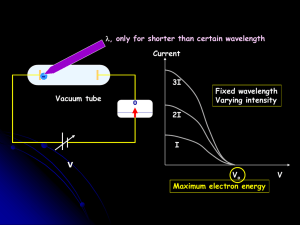
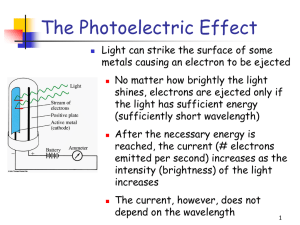
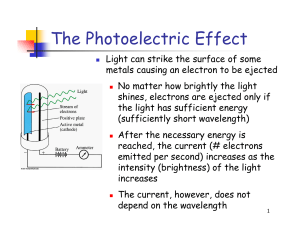
... Light consists of photons, each with a particular amount of energy, called a quantum of energy Upon collision, each photon can transfer its energy to a single electron The more photons strike the surface of the metal, the more electrons are liberated and the higher is the current ...
... Light consists of photons, each with a particular amount of energy, called a quantum of energy Upon collision, each photon can transfer its energy to a single electron The more photons strike the surface of the metal, the more electrons are liberated and the higher is the current ...
The Photoelectric Effect
... Light consists of photons, each with a particular amount of energy, called a quantum of energy Upon collision, each photon can transfer its energy to a single electron The more photons strike the surface of the metal, the more electrons are liberated and the higher is the current ...
... Light consists of photons, each with a particular amount of energy, called a quantum of energy Upon collision, each photon can transfer its energy to a single electron The more photons strike the surface of the metal, the more electrons are liberated and the higher is the current ...
Honors Chemistry
... The Development of Atomic Models Rutherford’s atomic model could not explain the chemical properties of elements. In 1913, Neils Bohr (Danish), stated that electrons occupy fixed paths or without giving off energy. Electrons far from the nucleus have ...
... The Development of Atomic Models Rutherford’s atomic model could not explain the chemical properties of elements. In 1913, Neils Bohr (Danish), stated that electrons occupy fixed paths or without giving off energy. Electrons far from the nucleus have ...
Mass number, atomic number and isotopes
... and 157Z Identify which of these atoms (not their real symbols) is a pair of isotopes. 3. For each element in question 2, state: ...
... and 157Z Identify which of these atoms (not their real symbols) is a pair of isotopes. 3. For each element in question 2, state: ...
Chapter 7 Many-Electron Atoms
... Electron spin is the cause of the fine structure in spectral lines, and the anomalous Zeeman effect ("extra" and "missing" splittings of spectral lines in the presence of magnetic fields). Electron spin is also important in magnetism. Spectral lines are due to photons emitted when electrons change t ...
... Electron spin is the cause of the fine structure in spectral lines, and the anomalous Zeeman effect ("extra" and "missing" splittings of spectral lines in the presence of magnetic fields). Electron spin is also important in magnetism. Spectral lines are due to photons emitted when electrons change t ...
History of the Atom
... - Beams bends away from the negatively charged plate and toward the positively charged plate. Concluded that a cathode ray consists of a beam of negatively charged particles (electrons). ...
... - Beams bends away from the negatively charged plate and toward the positively charged plate. Concluded that a cathode ray consists of a beam of negatively charged particles (electrons). ...
Heisenberg`s uncertainty principle
... quantum mechanics shows that certain pairs of physical properties, like position and speed, cannot both be known to arbitrary precision: the more precisely one property is known, the less precisely the other can be known. This statement is known as the uncertainty principle. The uncertainty principl ...
... quantum mechanics shows that certain pairs of physical properties, like position and speed, cannot both be known to arbitrary precision: the more precisely one property is known, the less precisely the other can be known. This statement is known as the uncertainty principle. The uncertainty principl ...
3.3 The Quantum Mechanical Model of the Atom
... there is always a node at each end •This means that there must be a whole number of half wavelengths in any of the allowed motions of the string ...
... there is always a node at each end •This means that there must be a whole number of half wavelengths in any of the allowed motions of the string ...
Electron

The electron is a subatomic particle, symbol e− or β−, with a negative elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value in units of ħ, which means that it is a fermion. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all matter, electrons have properties of both particles and waves, and so can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a higher De Broglie wavelength for typical energies.Many physical phenomena involve electrons in an essential role, such as electricity, magnetism, and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. An electron generates an electric field surrounding it. An electron moving relative to an observer generates a magnetic field. External magnetic fields deflect an electron. Electrons radiate or absorb energy in the form of photons when accelerated. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma using electromagnetic fields, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.Interactions involving electrons and other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between positive protons inside atomic nuclei and negative electrons composes atoms. Ionization or changes in the proportions of particles changes the binding energy of the system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms in 1838; Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.