Atomic Structure and Electron Configurations Multiple Choice PSI
... 28. The lowest orbital energy is reached when the number of electrons with the same spin is maximized. This statement describes __________. A. Pauli Exclusion Principle B. Hund’s Rule C. deBroglie hypothesis D. Heisenberg Uncertainty Principle 29. Which electron configuration correctly denotes an at ...
... 28. The lowest orbital energy is reached when the number of electrons with the same spin is maximized. This statement describes __________. A. Pauli Exclusion Principle B. Hund’s Rule C. deBroglie hypothesis D. Heisenberg Uncertainty Principle 29. Which electron configuration correctly denotes an at ...
Chapter 6: Electronic Structure of Atoms Recommended Text
... In what ways do the two isotopes differ from each other? Does the electronic configuration of 10B differ from that of 11B? Draw the orbital diagram for an atom of 11B. Which electrons are the valence electrons? Indicate three major ways in which the 1s electrons in boron differ from its 2s electrons ...
... In what ways do the two isotopes differ from each other? Does the electronic configuration of 10B differ from that of 11B? Draw the orbital diagram for an atom of 11B. Which electrons are the valence electrons? Indicate three major ways in which the 1s electrons in boron differ from its 2s electrons ...
Atomic Structure and Electron Configurations Multiple Choice PSI
... 28. The lowest orbital energy is reached when the number of electrons with the same spin is maximized. This statement describes __________. A. Pauli Exclusion Principle B. Hund’s Rule C. deBroglie hypothesis D. Heisenberg Uncertainty Principle 29. Which electron configuration correctly denotes an at ...
... 28. The lowest orbital energy is reached when the number of electrons with the same spin is maximized. This statement describes __________. A. Pauli Exclusion Principle B. Hund’s Rule C. deBroglie hypothesis D. Heisenberg Uncertainty Principle 29. Which electron configuration correctly denotes an at ...
Nuclear Chemistry - sullivanchem-ap
... 1. D—The mass should be 226 – (4 + 4 + 0 + 4) = 214. The atomic number should be 88 – (2 + 2 – 1 + 2) ...
... 1. D—The mass should be 226 – (4 + 4 + 0 + 4) = 214. The atomic number should be 88 – (2 + 2 – 1 + 2) ...
Lecture 5
... Pauli principle does not allow for two atomic electrons with the same quantum numbers, therefore each next electron will have to have at least one quantum number [n, ℓ, mℓ, ms ] different from all the other ones. We will use ↑ for ms=1/2 and ↓ for ms=-1/2. List of distinct sets of quantum number com ...
... Pauli principle does not allow for two atomic electrons with the same quantum numbers, therefore each next electron will have to have at least one quantum number [n, ℓ, mℓ, ms ] different from all the other ones. We will use ↑ for ms=1/2 and ↓ for ms=-1/2. List of distinct sets of quantum number com ...
1time/100kg day),producing atomic recoil, Direct detection of dark
... enclosing the milky way. The earth and solar system like a small fish, swimming in it. Dark Matter particle has a small probability hitting the atomic nuclei (<1time/100kg day), producing atomic recoil, ...
... enclosing the milky way. The earth and solar system like a small fish, swimming in it. Dark Matter particle has a small probability hitting the atomic nuclei (<1time/100kg day), producing atomic recoil, ...
7.4 The Wavelike properties of the Electron Models of
... properties – the probability to find a particle at a particular location depends on the amplitude (intensity) of the wave at this location ...
... properties – the probability to find a particle at a particular location depends on the amplitude (intensity) of the wave at this location ...
Assignment #1
... Modern Physics 3rd Ed. Serway, Moses, and Moyer- Chapter 1 #12, 20, 26, 27, 28, 31 Problem #7: A relativistic subatomic particle of mass m is moving away from the detector when it spontaneously decays, sending a photon toward the detector. The photon is observed to be red shifted by a factor of 100, ...
... Modern Physics 3rd Ed. Serway, Moses, and Moyer- Chapter 1 #12, 20, 26, 27, 28, 31 Problem #7: A relativistic subatomic particle of mass m is moving away from the detector when it spontaneously decays, sending a photon toward the detector. The photon is observed to be red shifted by a factor of 100, ...
General concepts of radiation
... It has been seen that the energy is carried by EMR in the form of discrete bundles or quanta which do not posses any mass. These quanta are often simply referred to as photons (because they were first investigated as an optical (light) phenomena). The energy transferred/carried by photons is greater ...
... It has been seen that the energy is carried by EMR in the form of discrete bundles or quanta which do not posses any mass. These quanta are often simply referred to as photons (because they were first investigated as an optical (light) phenomena). The energy transferred/carried by photons is greater ...
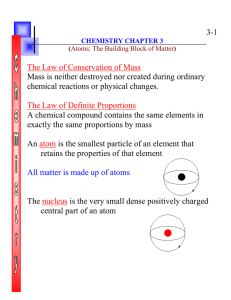
Chapter 3 Atoms: The Building Blocks
... chemical reactions or physical changes. The Law of Definite Proportions A chemical compound contains the same elements in exactly the same proportions by mass An atom is the smallest particle of an element that retains the properties of that element All matter is made up of atoms ...
... chemical reactions or physical changes. The Law of Definite Proportions A chemical compound contains the same elements in exactly the same proportions by mass An atom is the smallest particle of an element that retains the properties of that element All matter is made up of atoms ...
Electron Configuration Class Notes
... Energy moves in waves, but it can act as particles (photons). Louie de Broglie – “matter waves” Postulated that since light shows a “dual nature” – has wave properties as well as particulate properties, then matter should also be able to move - not only as particles - but also as waves! - this prope ...
... Energy moves in waves, but it can act as particles (photons). Louie de Broglie – “matter waves” Postulated that since light shows a “dual nature” – has wave properties as well as particulate properties, then matter should also be able to move - not only as particles - but also as waves! - this prope ...
What is electricity
... Since electrons are free to move from atom to atom some atoms are left with more protons than electrons making them positively charged atoms. ...
... Since electrons are free to move from atom to atom some atoms are left with more protons than electrons making them positively charged atoms. ...
Helium Atom
... well as in the near & far UV regions. There are twice as many line series as for the alkalis; two principal series in the visible and near UV, as well as two diffuse, two sharp and two fundamental series. This observation cannot be described by simple concept from hydrogen atom. When more than one e ...
... well as in the near & far UV regions. There are twice as many line series as for the alkalis; two principal series in the visible and near UV, as well as two diffuse, two sharp and two fundamental series. This observation cannot be described by simple concept from hydrogen atom. When more than one e ...
Double-Slit Experiment
... Curved (accelerated) motion means that electron should do what? = ASSUMPTIONS BASED IN CLASSICAL PHYSICS!!! Quantum physics: ...
... Curved (accelerated) motion means that electron should do what? = ASSUMPTIONS BASED IN CLASSICAL PHYSICS!!! Quantum physics: ...
electron-diffraction-tube-qrg
... emission at the heated cathode and focuses it into a thin beam by the control grid (or “Wehnelt cylinder”). ...
... emission at the heated cathode and focuses it into a thin beam by the control grid (or “Wehnelt cylinder”). ...
chapter 7: atomic structure and periodicity
... 17.3 The Atomic Spectrum of Hydrogen Continous spectrum – includes all ____________________ within a given range Line spectrum (also called emission spectrum) show only certain wavelengths. Line spectrum of hydrogen: The line spectrum of hydrogen indicated only certain ____________ are allowed for t ...
... 17.3 The Atomic Spectrum of Hydrogen Continous spectrum – includes all ____________________ within a given range Line spectrum (also called emission spectrum) show only certain wavelengths. Line spectrum of hydrogen: The line spectrum of hydrogen indicated only certain ____________ are allowed for t ...
Basic Physics Concepts Useful in Astronomy
... o An electron can go up a level via absorbing a photon (photon energy amount depends on what level it wants to go to) o An electron can go down a level by spitting out a photon (photon energy amount depends on what level it wants to go to) o Different atoms will only absorb and emit photons of certa ...
... o An electron can go up a level via absorbing a photon (photon energy amount depends on what level it wants to go to) o An electron can go down a level by spitting out a photon (photon energy amount depends on what level it wants to go to) o Different atoms will only absorb and emit photons of certa ...
Electron

The electron is a subatomic particle, symbol e− or β−, with a negative elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value in units of ħ, which means that it is a fermion. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all matter, electrons have properties of both particles and waves, and so can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a higher De Broglie wavelength for typical energies.Many physical phenomena involve electrons in an essential role, such as electricity, magnetism, and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. An electron generates an electric field surrounding it. An electron moving relative to an observer generates a magnetic field. External magnetic fields deflect an electron. Electrons radiate or absorb energy in the form of photons when accelerated. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma using electromagnetic fields, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.Interactions involving electrons and other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between positive protons inside atomic nuclei and negative electrons composes atoms. Ionization or changes in the proportions of particles changes the binding energy of the system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms in 1838; Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.