Electrons in Atoms
... Electrons of an element can absorb energy and emit the energy as EM radiation These emission spectra are not continuous ...
... Electrons of an element can absorb energy and emit the energy as EM radiation These emission spectra are not continuous ...
uncertainty, atom
... charge, and accelerating charges give off EM radiation (like an antenna), thus giving off energy. The electron would gradually lose all its energy. That doesn’t happen -- atoms are stable. ...
... charge, and accelerating charges give off EM radiation (like an antenna), thus giving off energy. The electron would gradually lose all its energy. That doesn’t happen -- atoms are stable. ...
File - Rogers` Rocket Science
... An ion is an atom or group of atoms that has a ________ or _________ charge. Remember that atoms are neutral because the # of protons = # of electrons. Some compounds are composed of particles called ions. Positive and negative ions are formed when __________ are __________ (lost or gained) ...
... An ion is an atom or group of atoms that has a ________ or _________ charge. Remember that atoms are neutral because the # of protons = # of electrons. Some compounds are composed of particles called ions. Positive and negative ions are formed when __________ are __________ (lost or gained) ...
THE ATOMIC NU
... (D40) in 1915 and is known as Duane and Hunt's law. b. Quantum-mechanical Theory of Bremsstrahlung. The deflection of a swift electron of velocity V = {3c, rest mass mo, by a nucleus of charge Ze falls in the domain of Z/137{3 « 1, if Z is not too large. This puts the interaction into the familiar " ...
... (D40) in 1915 and is known as Duane and Hunt's law. b. Quantum-mechanical Theory of Bremsstrahlung. The deflection of a swift electron of velocity V = {3c, rest mass mo, by a nucleus of charge Ze falls in the domain of Z/137{3 « 1, if Z is not too large. This puts the interaction into the familiar " ...
Parts of Unit 4 and 5Chp 5-6 – Electrons and
... You can find out where the electron is, but not where it is going. ...
... You can find out where the electron is, but not where it is going. ...
Chapter 4: Arrangement of Electrons in Atoms
... 1. Another problem that could not be explained by the wave theory of light was the line spectrum of the hydrogen atom. 2. A line spectrum is produced when a current is passed through a sample of hydrogen. The energy emitted by the sample is then passed through a prism. The resulting separation of li ...
... 1. Another problem that could not be explained by the wave theory of light was the line spectrum of the hydrogen atom. 2. A line spectrum is produced when a current is passed through a sample of hydrogen. The energy emitted by the sample is then passed through a prism. The resulting separation of li ...
Lec-23_Strachan
... Mendeleev arranged the elements according to their atomic masses and chemical similarities The electronic configuration of the elements explained by quantum numbers and Pauli’s Exclusion Principle explains the configuration ...
... Mendeleev arranged the elements according to their atomic masses and chemical similarities The electronic configuration of the elements explained by quantum numbers and Pauli’s Exclusion Principle explains the configuration ...
File
... electron: particle that has a negative (-) charge ~ very small ~ about 1/1800th the size of a proton or neutron atoms have no overall charge (neutral) because there is an equal number of protons (+) and electrons (-) ...
... electron: particle that has a negative (-) charge ~ very small ~ about 1/1800th the size of a proton or neutron atoms have no overall charge (neutral) because there is an equal number of protons (+) and electrons (-) ...
Word Format
... To determine the impulse, we need to determine the time that the force interacts. We will assume that the force stays at its maximum value for the time required for the alpha to travel across the diameter of the gold atom. This is an approximation but it should over-estimate the alpha deflection and ...
... To determine the impulse, we need to determine the time that the force interacts. We will assume that the force stays at its maximum value for the time required for the alpha to travel across the diameter of the gold atom. This is an approximation but it should over-estimate the alpha deflection and ...
Read Notes #1
... To determine the impulse, we need to determine the time that the force interacts. We will assume that the force stays at its maximum value for the time required for the alpha to travel across the diameter of the gold atom. This is an approximation but it should over-estimate the alpha deflection and ...
... To determine the impulse, we need to determine the time that the force interacts. We will assume that the force stays at its maximum value for the time required for the alpha to travel across the diameter of the gold atom. This is an approximation but it should over-estimate the alpha deflection and ...
4.2 - Science with Mrs. Vaness
... – He concluded that all the ____________ charge and almost all of the mass are concentrated in a _________ region that has enough positive charge to account for the great _____________ of some of the alpha particles. The Rutherford atomic model is known as the ____________ _________. – In the nuclea ...
... – He concluded that all the ____________ charge and almost all of the mass are concentrated in a _________ region that has enough positive charge to account for the great _____________ of some of the alpha particles. The Rutherford atomic model is known as the ____________ _________. – In the nuclea ...
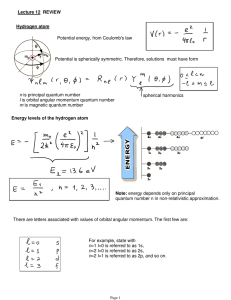
Lecture 12: Review.
... effective magnetic field the electrons see due to orbital motion around the nucleus. ...
... effective magnetic field the electrons see due to orbital motion around the nucleus. ...
Exam 1 Topics to Review (McMurry Chpts 1
... 2. Label each element below as transition metal, main group metal, halogen, noble gas, nonmetal (more than one description may apply for each element): ...
... 2. Label each element below as transition metal, main group metal, halogen, noble gas, nonmetal (more than one description may apply for each element): ...
GROUP QUIZ UNIT 04 NAMES I. Fill in the charts (1 point per blank
... a. the 2s orbital can hold more electrons. b. the 2s orbital has a slightly different shape. c. the 2s orbital is at a higher energy level. d. the 1s orbital can have only one electron. ____18. The maximum number of electrons that can occupy any single orbital at any energy level is a. two, if they ...
... a. the 2s orbital can hold more electrons. b. the 2s orbital has a slightly different shape. c. the 2s orbital is at a higher energy level. d. the 1s orbital can have only one electron. ____18. The maximum number of electrons that can occupy any single orbital at any energy level is a. two, if they ...
How are quantum numbers used to describe electrons
... How many orbitals in the 4th energy level? How many electrons can be in the 4th energy level? For the known elements, ________ orbitals and ______ electrons is the maximum number in energy levels 5-7. What rules are used to explain how electrons fill orbitals? Pauli exclusion principle—no two electr ...
... How many orbitals in the 4th energy level? How many electrons can be in the 4th energy level? For the known elements, ________ orbitals and ______ electrons is the maximum number in energy levels 5-7. What rules are used to explain how electrons fill orbitals? Pauli exclusion principle—no two electr ...
E - Department of Physics
... Example: Nanotechnology for shaping electron waves. 2) Quantum physics is important for large energy quanta E = h f : Example: Planck’s radiation law cuts the spectrum off when the energy to create a photon exceeds the available thermal energy ( Etherm 0.1 eV at T=300K ) : E > Etherm Example: The ...
... Example: Nanotechnology for shaping electron waves. 2) Quantum physics is important for large energy quanta E = h f : Example: Planck’s radiation law cuts the spectrum off when the energy to create a photon exceeds the available thermal energy ( Etherm 0.1 eV at T=300K ) : E > Etherm Example: The ...
Calculating particle properties of a wave
... Example: Nanotechnology for shaping electron waves. 2) Quantum physics is important for large energy quanta E = h f : Example: Planck’s radiation law cuts the spectrum off when the energy to create a photon exceeds the available thermal energy ( Etherm 0.1 eV at T=300K ) : E > Etherm Example: The ...
... Example: Nanotechnology for shaping electron waves. 2) Quantum physics is important for large energy quanta E = h f : Example: Planck’s radiation law cuts the spectrum off when the energy to create a photon exceeds the available thermal energy ( Etherm 0.1 eV at T=300K ) : E > Etherm Example: The ...
clasPoster5 - University of Richmond
... • Replace current mini-torus for Moller electron shielding. • Provide magnetic field for large angle momentum analysis. ...
... • Replace current mini-torus for Moller electron shielding. • Provide magnetic field for large angle momentum analysis. ...
Electron

The electron is a subatomic particle, symbol e− or β−, with a negative elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value in units of ħ, which means that it is a fermion. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all matter, electrons have properties of both particles and waves, and so can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a higher De Broglie wavelength for typical energies.Many physical phenomena involve electrons in an essential role, such as electricity, magnetism, and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. An electron generates an electric field surrounding it. An electron moving relative to an observer generates a magnetic field. External magnetic fields deflect an electron. Electrons radiate or absorb energy in the form of photons when accelerated. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma using electromagnetic fields, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.Interactions involving electrons and other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between positive protons inside atomic nuclei and negative electrons composes atoms. Ionization or changes in the proportions of particles changes the binding energy of the system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms in 1838; Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.