File - Rogers` Rocket Science
... tend to LOSE electrons, from their outer energy level • Sodium loses one: there are now more protons (11) than electrons (10), and thus a positively charged particle is formed = “cation” • The charge is written as a number followed by a plus sign: Na1+ • Na1+ is re-named a sodium ion ...
... tend to LOSE electrons, from their outer energy level • Sodium loses one: there are now more protons (11) than electrons (10), and thus a positively charged particle is formed = “cation” • The charge is written as a number followed by a plus sign: Na1+ • Na1+ is re-named a sodium ion ...
SPATIAL EXTENSIONS AND MAGNETIC MOMENTUM OF THE
... protons and electrons, show that the proton is able to be inhoused into the limits of an electron. The electron, as well as the proton, are supposed being torus particles, having radii, R , and , r , respectively, where , R , is the radius associated to a main spin axis and , r , is associated to th ...
... protons and electrons, show that the proton is able to be inhoused into the limits of an electron. The electron, as well as the proton, are supposed being torus particles, having radii, R , and , r , respectively, where , R , is the radius associated to a main spin axis and , r , is associated to th ...
CH17 Self Assessment
... To meet an excellent standard I will also be able to: use hand rule to explain how to determine nature of charge or direction of magnetic field given the other and the track for subatomic particles compare tracks in terms of the mass, charge (size or nature), speed, or energy of the particle compare ...
... To meet an excellent standard I will also be able to: use hand rule to explain how to determine nature of charge or direction of magnetic field given the other and the track for subatomic particles compare tracks in terms of the mass, charge (size or nature), speed, or energy of the particle compare ...
Element: pure substances that are made up of one kind of atom
... Nucleus: located at the center of the atom. Consists of protons and neutrons. Electrons surround the nucleus. Electron Cloud: the current model of an atom contains an electron cloud that shows electrons traveling in specific energy levels around a nucleus. The farther away from the nucleus an electr ...
... Nucleus: located at the center of the atom. Consists of protons and neutrons. Electrons surround the nucleus. Electron Cloud: the current model of an atom contains an electron cloud that shows electrons traveling in specific energy levels around a nucleus. The farther away from the nucleus an electr ...
$doc.title
... OK irrespective of signs of A or B But if exchange once, for A +ve, B could be +ve or –ve…. Reality: It’s –ve for Fermions and +ve for Bosons! Means that if we try to get fermions 1 and 2 into same state then ψ → 0 !!!! This is the Pauli Exclusion Principle: No two fermions (electrons) can h ...
... OK irrespective of signs of A or B But if exchange once, for A +ve, B could be +ve or –ve…. Reality: It’s –ve for Fermions and +ve for Bosons! Means that if we try to get fermions 1 and 2 into same state then ψ → 0 !!!! This is the Pauli Exclusion Principle: No two fermions (electrons) can h ...
Electricity Unit Notes: pp
... c) __________- the electrical potential of electrons (measured in Volts) d) Electrons flow through a wire very slowly. e) __________ work when the positive end is connected to the negative end (where the electrons are until the circuit is completed) via a wire as seen in the figure above. i) The che ...
... c) __________- the electrical potential of electrons (measured in Volts) d) Electrons flow through a wire very slowly. e) __________ work when the positive end is connected to the negative end (where the electrons are until the circuit is completed) via a wire as seen in the figure above. i) The che ...
_____ Name _____ _ Date ______ Mrs. G
... Which 2 subatomic particles make up most of the mass of atoms? Which subatomic particle has negligible mass? o Why do atoms found in their neutral state have no charge? o What would happen if an atom gained an electron? o What would happen if an atom lost an electron? o What do we call an atom t ...
... Which 2 subatomic particles make up most of the mass of atoms? Which subatomic particle has negligible mass? o Why do atoms found in their neutral state have no charge? o What would happen if an atom gained an electron? o What would happen if an atom lost an electron? o What do we call an atom t ...
Plum pudding
... Problem 1a.- The plum pudding analogy hides the fact that Thomson thought the electrons were moving inside the atom. Nevertheless let’s consider a plum pudding model of a lithium ion (Li+): A spherical volume of radius R with homogeneously distributed positive charge = +3e and two electrons, with ch ...
... Problem 1a.- The plum pudding analogy hides the fact that Thomson thought the electrons were moving inside the atom. Nevertheless let’s consider a plum pudding model of a lithium ion (Li+): A spherical volume of radius R with homogeneously distributed positive charge = +3e and two electrons, with ch ...
Chapter 4.2 Notes
... an ________. However, we do have microscope that can show how _____________ are arranged on the _____________ of a material. 6. Atomic Number and Mass Number A. The ____________ number of an element equals the number of _______________ in an _______________ of that element. B. The ___________ of any ...
... an ________. However, we do have microscope that can show how _____________ are arranged on the _____________ of a material. 6. Atomic Number and Mass Number A. The ____________ number of an element equals the number of _______________ in an _______________ of that element. B. The ___________ of any ...
Chapter 2.2 and 7 Notes
... 5 orbitals, 2 electrons each 10 total electrons in each energy level Do not show up until the 3rd energy level ...
... 5 orbitals, 2 electrons each 10 total electrons in each energy level Do not show up until the 3rd energy level ...
Topic 4: Materials - Education Umbrella
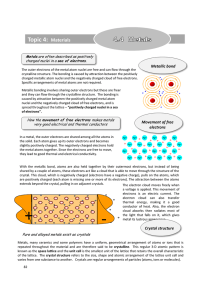
... the solid. Each atom gives up its outer electrons and becomes slightly positively charged. The negatively charged electrons hold the metal atoms together. Since the electrons are free to move, they lead to good thermal and electrical conductivity. ...
... the solid. Each atom gives up its outer electrons and becomes slightly positively charged. The negatively charged electrons hold the metal atoms together. Since the electrons are free to move, they lead to good thermal and electrical conductivity. ...
Chemistry Review - Net Start Class
... • Atoms of substances rearrange themselves into a new substance. • The Reactants combine to form new Products ...
... • Atoms of substances rearrange themselves into a new substance. • The Reactants combine to form new Products ...
The Bohr model for the electrons
... Life at the electron level is very different Key to unlocking the low door to the secret garden of the atom lay in accepting the wave properties of electrons ...
... Life at the electron level is very different Key to unlocking the low door to the secret garden of the atom lay in accepting the wave properties of electrons ...
For this basic module we simply take the suitable module
... Basic mechanics yields for a single particle with momentum p F = dp/dt = m·dv/dt with p = momentum of the electron. Note that p does not have to be zero when the field is switched on. If this would be all, the velocity of a given electron would acquire an ever increasing component in field direction ...
... Basic mechanics yields for a single particle with momentum p F = dp/dt = m·dv/dt with p = momentum of the electron. Note that p does not have to be zero when the field is switched on. If this would be all, the velocity of a given electron would acquire an ever increasing component in field direction ...
Quiz 3-6 fy13 - Nuclear Chemistry practice
... D. loses energy by emitting radiation E. all of the above ...
... D. loses energy by emitting radiation E. all of the above ...
photoelectric effect Work function
... up, we may say that there is hardly one among the great problems, in which modern ...
... up, we may say that there is hardly one among the great problems, in which modern ...
13 ELECTRONS IN ATOMS
... 13. Use the diagram above. Describe how the px , py , and pz orbitals are similar. The p orbitals are similar because they are all dumbbell shaped. The p orbitals have different 14. Describe how the px , py , and pz orbitals are different. _____________________________ orientations in space. They ar ...
... 13. Use the diagram above. Describe how the px , py , and pz orbitals are similar. The p orbitals are similar because they are all dumbbell shaped. The p orbitals have different 14. Describe how the px , py , and pz orbitals are different. _____________________________ orientations in space. They ar ...
electron cloud - Wickliffe City School
... Nonmetals are are electron takers and have high electronegativities. What about the noble gases? ...
... Nonmetals are are electron takers and have high electronegativities. What about the noble gases? ...
Unit 6 Science Vocabulary Emily 6th
... 5. an arrangement of the elements in order of their atomic numbers such that elements with similar properties fall in the same group 6. the smallest particle into which an element can be divided. 7. uncharged particles that are found within the nucleus of an atom. 8. the center of an atom made up of ...
... 5. an arrangement of the elements in order of their atomic numbers such that elements with similar properties fall in the same group 6. the smallest particle into which an element can be divided. 7. uncharged particles that are found within the nucleus of an atom. 8. the center of an atom made up of ...
Electron

The electron is a subatomic particle, symbol e− or β−, with a negative elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value in units of ħ, which means that it is a fermion. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all matter, electrons have properties of both particles and waves, and so can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a higher De Broglie wavelength for typical energies.Many physical phenomena involve electrons in an essential role, such as electricity, magnetism, and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. An electron generates an electric field surrounding it. An electron moving relative to an observer generates a magnetic field. External magnetic fields deflect an electron. Electrons radiate or absorb energy in the form of photons when accelerated. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma using electromagnetic fields, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.Interactions involving electrons and other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between positive protons inside atomic nuclei and negative electrons composes atoms. Ionization or changes in the proportions of particles changes the binding energy of the system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms in 1838; Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.