12.50, £6.25. GA Jones. Pp. 175. London" Edward
... vast amount of existing information about electron microscopy and its applications, with special emphasis on the problems in solid-state physics. The resulting book contains fundamental information about the principles on which electron microscopy is based, the design and construction of electron mi ...
... vast amount of existing information about electron microscopy and its applications, with special emphasis on the problems in solid-state physics. The resulting book contains fundamental information about the principles on which electron microscopy is based, the design and construction of electron mi ...
orbital
... be any integer between 0 and n - 1. For n = 3, l can be either 0, 1, or 2. The magnetic quantum number (m) can be any integer between -l and +l. For l = 2, m can be either -2, -1, 0, +1, or ...
... be any integer between 0 and n - 1. For n = 3, l can be either 0, 1, or 2. The magnetic quantum number (m) can be any integer between -l and +l. For l = 2, m can be either -2, -1, 0, +1, or ...
Electron Configurations - Birmingham City Schools
... only hydrogen). Since it couldn't predict the chemical properties (ionization energy, for example) for anything other than hydrogen, it had to be modified. Other scientists found discrepancies in the trend in ionization energy. Others found details in the spectra (remember Bohr's model came from ele ...
... only hydrogen). Since it couldn't predict the chemical properties (ionization energy, for example) for anything other than hydrogen, it had to be modified. Other scientists found discrepancies in the trend in ionization energy. Others found details in the spectra (remember Bohr's model came from ele ...
Physical Science Worksheet: History of the Periodic Table Short
... 1. How did chemists change Mendeleev’s periodic table in the early 1900s? 2. What prediction did Mendeleev make that came true less than 20 years later? 3. Phosphorus-33 (atomic number 15) contains how many electrons, protons, and neutrons? 4. A(n) _____ is an atom, or bonded group of atoms, that ha ...
... 1. How did chemists change Mendeleev’s periodic table in the early 1900s? 2. What prediction did Mendeleev make that came true less than 20 years later? 3. Phosphorus-33 (atomic number 15) contains how many electrons, protons, and neutrons? 4. A(n) _____ is an atom, or bonded group of atoms, that ha ...
Chapter 6 Outline full
... Heisenberg’s uncertainty principle: We cannot determine the exact position, direction of motion, and speed of subatomic particles simultaneously. ...
... Heisenberg’s uncertainty principle: We cannot determine the exact position, direction of motion, and speed of subatomic particles simultaneously. ...
The Atoms Test Study Guide
... ATOMS TEST – STUDY GUIDE Test is on _____________ The History of Atomic Theory Develop. of Atomic Theory: Democritus to Thomson 1. Who first proposed that matter could not be divided forever? ___________ 2. What is an atom? ____________________________________________ 3. What particle did Thomson ...
... ATOMS TEST – STUDY GUIDE Test is on _____________ The History of Atomic Theory Develop. of Atomic Theory: Democritus to Thomson 1. Who first proposed that matter could not be divided forever? ___________ 2. What is an atom? ____________________________________________ 3. What particle did Thomson ...
Quantum Numbers, Orbitals, Electron Configurations, Periodic Trends
... Quantum Numbers, Orbitals, Electron Configurations, Periodic Trends CH2000: Introduction to General Chemistry, Plymouth State University, Fall 2014 1. Briefly describe in your own terms what each of the quantum numbers mean: n (principle q.n.) _____________________________________ ℓ (angular momentu ...
... Quantum Numbers, Orbitals, Electron Configurations, Periodic Trends CH2000: Introduction to General Chemistry, Plymouth State University, Fall 2014 1. Briefly describe in your own terms what each of the quantum numbers mean: n (principle q.n.) _____________________________________ ℓ (angular momentu ...
Lecture 4
... 3. If it is degenerate, how many states have the same energy and what are their quantum numbers ? (ignore spin) Answers ...
... 3. If it is degenerate, how many states have the same energy and what are their quantum numbers ? (ignore spin) Answers ...
Quantum Numbers, Orbitals, Electron Configurations, Periodic Trends
... Quantum Numbers, Orbitals, Electron Configurations, Periodic Trends CH2000: Introduction to General Chemistry, Plymouth State University, Fall 2013 1. Briefly describe in your own terms what each of the quantum numbers mean: n (principle q.n.) _____________________________________ ℓ (angular momentu ...
... Quantum Numbers, Orbitals, Electron Configurations, Periodic Trends CH2000: Introduction to General Chemistry, Plymouth State University, Fall 2013 1. Briefly describe in your own terms what each of the quantum numbers mean: n (principle q.n.) _____________________________________ ℓ (angular momentu ...
Problem Set 1 (Due January 30th by 7:00 PM) Answers to the
... Energy, Electrons and Periodic Trends (Bonus Date: January 26th) For all periodic trend problems, make sure that you can justify your answer. You will be expected to do this on an exam. 14. For each group of atoms, determine which would have a higher 1st Ionization Energy. a. Xe, Kr, Ar b. As, Cl, B ...
... Energy, Electrons and Periodic Trends (Bonus Date: January 26th) For all periodic trend problems, make sure that you can justify your answer. You will be expected to do this on an exam. 14. For each group of atoms, determine which would have a higher 1st Ionization Energy. a. Xe, Kr, Ar b. As, Cl, B ...
Improved sensitivity to the electron`s electric dipole
... It is well known that the electron has a magnetic dipole moment. This property has been measured with exquisite precision and provides one of the most successful comparisons between experiment and theory. However, no one has ever been able to detect the analogous electric dipole moment (EDM), de . I ...
... It is well known that the electron has a magnetic dipole moment. This property has been measured with exquisite precision and provides one of the most successful comparisons between experiment and theory. However, no one has ever been able to detect the analogous electric dipole moment (EDM), de . I ...
Correlation of Aqueous Redox Potentials with Gaseous Ionization
... Water plays an important role in many terrestrial and atmospheric processes as a mediator of electron transfer between gaseous species. In a recent investigation of the relation between aqueous standard redox potentials and gaseous ionization potentials, simple linear dependences were discovered for ...
... Water plays an important role in many terrestrial and atmospheric processes as a mediator of electron transfer between gaseous species. In a recent investigation of the relation between aqueous standard redox potentials and gaseous ionization potentials, simple linear dependences were discovered for ...
Chapter 28 Atoms
... The energy of an orbiting electron in an atom is the sum of the kinetic energy of the electron and the potential energy resulting from the attractive force between the electron and the nucleus. The energy of an electron in an orbit near the nucleus is less than that of an electron in an orbit farthe ...
... The energy of an orbiting electron in an atom is the sum of the kinetic energy of the electron and the potential energy resulting from the attractive force between the electron and the nucleus. The energy of an electron in an orbit near the nucleus is less than that of an electron in an orbit farthe ...
Lecture 19 - Guelph Physics
... have to worry about the acceleration and radiation associated with bending the path of the electron). Finally, scattering experiments with electrons have failed to identify any finite electron radius, it is as though they really are point particles. These experimental observations are difficult to r ...
... have to worry about the acceleration and radiation associated with bending the path of the electron). Finally, scattering experiments with electrons have failed to identify any finite electron radius, it is as though they really are point particles. These experimental observations are difficult to r ...
Motion Along a Straight Line at Constant
... which emits electrons, a nearby positive anode attracts these electrons which pass through a hole in the anode to form a beam. This is called Thermionic emission. The potential difference between the anode and cathode controls the speed of the electrons. ...
... which emits electrons, a nearby positive anode attracts these electrons which pass through a hole in the anode to form a beam. This is called Thermionic emission. The potential difference between the anode and cathode controls the speed of the electrons. ...
The Periodic table
... In 1926 Schrodinger showed that laws of quantum mechanics could be used to characterize the motion of electrons. A quantized property is a property that can have only certain values. The energy of an electron is quantized, only certain behavior patterns are allowed. ...
... In 1926 Schrodinger showed that laws of quantum mechanics could be used to characterize the motion of electrons. A quantized property is a property that can have only certain values. The energy of an electron is quantized, only certain behavior patterns are allowed. ...
Atomic History and Structure Atomic Timeline Dalton (Indivisible
... Nucleus: Protons - decides identity of atom Neutrons - hold protons in nucleus together Protons and Neutrons are a type of subatomic particle called a Hadron. Hadrons are composed of quarks (elementary particles/fermions that make up matter) and gluons (elementary particles/fermions that hold quarks ...
... Nucleus: Protons - decides identity of atom Neutrons - hold protons in nucleus together Protons and Neutrons are a type of subatomic particle called a Hadron. Hadrons are composed of quarks (elementary particles/fermions that make up matter) and gluons (elementary particles/fermions that hold quarks ...
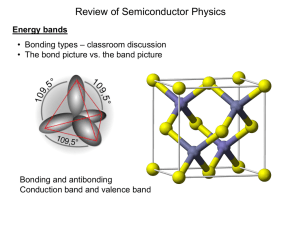
ECE692 Slides 3: Solid State Physics (Updated 09/18 - UTK-EECS
... Block electron ħk is the crystal momentum, which is not a momentum, but is treated as momentum in the semiclassical theory. n is the band index. 2 | k k 0 |2 E (k ) En(k) = En(k+K) ...
... Block electron ħk is the crystal momentum, which is not a momentum, but is treated as momentum in the semiclassical theory. n is the band index. 2 | k k 0 |2 E (k ) En(k) = En(k+K) ...
Electron

The electron is a subatomic particle, symbol e− or β−, with a negative elementary electric charge. Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they have no known components or substructure. The electron has a mass that is approximately 1/1836 that of the proton. Quantum mechanical properties of the electron include an intrinsic angular momentum (spin) of a half-integer value in units of ħ, which means that it is a fermion. Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle. Like all matter, electrons have properties of both particles and waves, and so can collide with other particles and can be diffracted like light. The wave properties of electrons are easier to observe with experiments than those of other particles like neutrons and protons because electrons have a lower mass and hence a higher De Broglie wavelength for typical energies.Many physical phenomena involve electrons in an essential role, such as electricity, magnetism, and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions. An electron generates an electric field surrounding it. An electron moving relative to an observer generates a magnetic field. External magnetic fields deflect an electron. Electrons radiate or absorb energy in the form of photons when accelerated. Laboratory instruments are capable of containing and observing individual electrons as well as electron plasma using electromagnetic fields, whereas dedicated telescopes can detect electron plasma in outer space. Electrons have many applications, including electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.Interactions involving electrons and other subatomic particles are of interest in fields such as chemistry and nuclear physics. The Coulomb force interaction between positive protons inside atomic nuclei and negative electrons composes atoms. Ionization or changes in the proportions of particles changes the binding energy of the system. The exchange or sharing of the electrons between two or more atoms is the main cause of chemical bonding. British natural philosopher Richard Laming first hypothesized the concept of an indivisible quantity of electric charge to explain the chemical properties of atoms in 1838; Irish physicist George Johnstone Stoney named this charge 'electron' in 1891, and J. J. Thomson and his team of British physicists identified it as a particle in 1897. Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are known as beta particles. Electrons may be created through beta decay of radioactive isotopes and in high-energy collisions, for instance when cosmic rays enter the atmosphere. The antiparticle of the electron is called the positron; it is identical to the electron except that it carries electrical and other charges of the opposite sign. When an electron collides with a positron, both particles may be totally annihilated, producing gamma ray photons.