PPT | 345.5 KB - Joint Quantum Institute
... Ordinarily, quantum dots (microscopic formations of semiconductor material) cannot form entangled pairs of indistinguishable photons because the processes involved in emission produce photons of slightly different wavelengths. The researchers found, however, that by beaming a carefully tuned laser a ...
... Ordinarily, quantum dots (microscopic formations of semiconductor material) cannot form entangled pairs of indistinguishable photons because the processes involved in emission produce photons of slightly different wavelengths. The researchers found, however, that by beaming a carefully tuned laser a ...
Mar 11/02 Matter Waves
... of the amplitude of the wave’s electric field in that region • Postulate that light travels not as a stream of photons but as a probability wave • photons only manifest themselves when light interacts with matter • photons originate in the source that produces the light wave (interaction) • photons ...
... of the amplitude of the wave’s electric field in that region • Postulate that light travels not as a stream of photons but as a probability wave • photons only manifest themselves when light interacts with matter • photons originate in the source that produces the light wave (interaction) • photons ...
Mechanisms for the Radiation of Electromagnetic Waves
... The temperature of an object is determined by the energy of the motion (called kinetic energy) of the objects in the system ...
... The temperature of an object is determined by the energy of the motion (called kinetic energy) of the objects in the system ...
File
... The energy levels are not equally spaced like a ladder – they get closer the farther from the nucleus you go The higher the energy of the e-, the easier it leaves the atom ...
... The energy levels are not equally spaced like a ladder – they get closer the farther from the nucleus you go The higher the energy of the e-, the easier it leaves the atom ...
The Chemical Basis of Life
... Anything that occupies space. Composed of one or more chemical elements. ...
... Anything that occupies space. Composed of one or more chemical elements. ...
Interactions
... electrons binding energy but also the average kinetic energy of the ejected electron and the average energy lost as incident particles excite atoms, interact with nuclei, and increase the rate of vibration of nearby molecules. ...
... electrons binding energy but also the average kinetic energy of the ejected electron and the average energy lost as incident particles excite atoms, interact with nuclei, and increase the rate of vibration of nearby molecules. ...
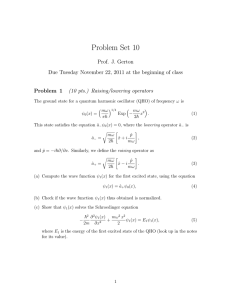
Problem Set 10
... components of the wavefunction? Why? (b) Write down the wave function for x > 0. Here, are there left- and right-moving components of the wavefunction? Why? (c) Write down the boundary conditions at x = 0 and solve for the amplitude coefficients of the reflected and transmitted waves, in terms of th ...
... components of the wavefunction? Why? (b) Write down the wave function for x > 0. Here, are there left- and right-moving components of the wavefunction? Why? (c) Write down the boundary conditions at x = 0 and solve for the amplitude coefficients of the reflected and transmitted waves, in terms of th ...
Review Notes - Biochemistry
... The goal of all atoms is to have a _STABLE_ outer energy level. The goal leads to bonding of atoms. 2 types of bonding: 1. Ionic Bonding: When _1_ or more electrons are _TRANSFERRED_ from one atom to another. Ion: an atom with a_CHARGE_. When an electron is gained it will be _NEGATIVE_charged ...
... The goal of all atoms is to have a _STABLE_ outer energy level. The goal leads to bonding of atoms. 2 types of bonding: 1. Ionic Bonding: When _1_ or more electrons are _TRANSFERRED_ from one atom to another. Ion: an atom with a_CHARGE_. When an electron is gained it will be _NEGATIVE_charged ...
Radiation for Radionuclide Users
... while the plutonium-beryllium (PuBe) sources and the americiumberyllium (AmBe) sources only produce small amounts of very low energy gamma radiation. Thus, as neutron sources, PuBe and AmBe sources tend to be less hazardous to handle. The older RaBe sources also had a tendency to develop leaks over ...
... while the plutonium-beryllium (PuBe) sources and the americiumberyllium (AmBe) sources only produce small amounts of very low energy gamma radiation. Thus, as neutron sources, PuBe and AmBe sources tend to be less hazardous to handle. The older RaBe sources also had a tendency to develop leaks over ...