topic-2.doc
... Electrons are in orbit around the nucleus, are involved in chemical reactions. o Orbital: three-dimensional space where an electron will most likely be found 90% of the time o First energy level: one s orbital, holds 2 electrons o Second energy level: one s and three p orbitals, holds 8 electrons Ch ...
... Electrons are in orbit around the nucleus, are involved in chemical reactions. o Orbital: three-dimensional space where an electron will most likely be found 90% of the time o First energy level: one s orbital, holds 2 electrons o Second energy level: one s and three p orbitals, holds 8 electrons Ch ...
PPT
... • Light shining on a metal can “knock” electrons out of atoms. • Light must provide energy to overcome Coulomb attraction of electron to nucleus • Light Intensity gives power/area (i.e. Watts/m2) – Recall: Power = Energy/time (i.e. Joules/sec.) light ...
... • Light shining on a metal can “knock” electrons out of atoms. • Light must provide energy to overcome Coulomb attraction of electron to nucleus • Light Intensity gives power/area (i.e. Watts/m2) – Recall: Power = Energy/time (i.e. Joules/sec.) light ...
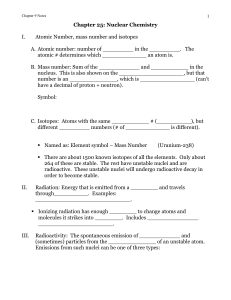
Chapter 9: Nuclear Chemistry
... different _________ numbers (# of _____________ is different). Named as: Element symbol – Mass Number ...
... different _________ numbers (# of _____________ is different). Named as: Element symbol – Mass Number ...
pacing guide - Tallapoosa County Schools
... the Doppler effect. Explaining reasons for differences in speed, frequency, and wavelength of a propagating wave in varying materials Describing uses of different components of the electromagnetic spectrum, including radio waves, microwaves, infrared radiation, visible light, ultraviolet radiati ...
... the Doppler effect. Explaining reasons for differences in speed, frequency, and wavelength of a propagating wave in varying materials Describing uses of different components of the electromagnetic spectrum, including radio waves, microwaves, infrared radiation, visible light, ultraviolet radiati ...
22.2 Production of Electromagnetic Waves Oscillating charges will
... Nature of Electromagnetic Radiation The success of Maxwell’s Equation appeared to be clear proof that light was a wave phenomena, but we will see in Ch 27 that Einstein suggested that light had a dual naturesome experiments show wave properties and others show particle properties. •For wave propert ...
... Nature of Electromagnetic Radiation The success of Maxwell’s Equation appeared to be clear proof that light was a wave phenomena, but we will see in Ch 27 that Einstein suggested that light had a dual naturesome experiments show wave properties and others show particle properties. •For wave propert ...
HNRS 227 Lecture #2 Chapters 2 and 3
... What was so unusual about Planck’s findings about blackbody radiation? Why was this considered revolutionary? Planck's findings were revolutionary because they meant that vibrating molecules could only have a fixed amount of energy that could only be multiples of a certain amount called the quant ...
... What was so unusual about Planck’s findings about blackbody radiation? Why was this considered revolutionary? Planck's findings were revolutionary because they meant that vibrating molecules could only have a fixed amount of energy that could only be multiples of a certain amount called the quant ...
modern mini test Jan 2011
... a) What is the half-life of the radioisotope? b) How much time is required (from the original 320 g sample) for the mass of the remaining radioisotope to decrease to 5 g? 2. A particle has a de Broglie wavelength of 6.8 1014 m. Calculate the mass of the particle if it is travelling at a speed of ...
... a) What is the half-life of the radioisotope? b) How much time is required (from the original 320 g sample) for the mass of the remaining radioisotope to decrease to 5 g? 2. A particle has a de Broglie wavelength of 6.8 1014 m. Calculate the mass of the particle if it is travelling at a speed of ...
Problem set 4
... 900 Watts in a collimated beam in the x̂ direction. What is the force on the source? h2i 2. How many photons from a 100 MHz beam of FM radio waves must an electron absorb before it has gained an energy of 10 eV? h1i 3. Is the discreteness of the energy in an electromagnetic wave more easily detected ...
... 900 Watts in a collimated beam in the x̂ direction. What is the force on the source? h2i 2. How many photons from a 100 MHz beam of FM radio waves must an electron absorb before it has gained an energy of 10 eV? h1i 3. Is the discreteness of the energy in an electromagnetic wave more easily detected ...