RedOx notes:
... Which elements have specific rules? Which element(s) do(es) not have rules? Use rule 8 or 9 from above to calculate these. ...
... Which elements have specific rules? Which element(s) do(es) not have rules? Use rule 8 or 9 from above to calculate these. ...
Assigning and Using Oxidation Numbers in Biochemistry Lecture
... assigning oxidation numbers to metabolic intermediates and end products in biochemical equations. Oxidation–reduction reactions, reactions in which electrons are transferred, comprise a major class of biochemical processes. For example, why anaerobic metabolism produces some products but cannot prod ...
... assigning oxidation numbers to metabolic intermediates and end products in biochemical equations. Oxidation–reduction reactions, reactions in which electrons are transferred, comprise a major class of biochemical processes. For example, why anaerobic metabolism produces some products but cannot prod ...
Lecture: 27 Fatty acid and triacyl glycerol biosynthesis Biosynthesis
... i. Synthesis takes place in the cytosol, in contrast with degradation or oxidation, which occurs in the mitochondrial matrix. ii. Intermediates in fatty acid synthesis are covalently linked to the sulfhydryl group of an acyl carrier protein (ACP) whereas intermediates in fatty acid breakdown are bon ...
... i. Synthesis takes place in the cytosol, in contrast with degradation or oxidation, which occurs in the mitochondrial matrix. ii. Intermediates in fatty acid synthesis are covalently linked to the sulfhydryl group of an acyl carrier protein (ACP) whereas intermediates in fatty acid breakdown are bon ...
The Permeability Properties of Rat Liver Lysosomes to Nucleosides
... digestion are the mononucleotides, which could be further degraded within lysosomes by an enzyme or enzymes of the acid phosphatase complex to yield the nucleosides (Arsenis e t ul., 1970). The fate of nucleosides arising within lysosomes in this way is not known. One possibility is the penetration ...
... digestion are the mononucleotides, which could be further degraded within lysosomes by an enzyme or enzymes of the acid phosphatase complex to yield the nucleosides (Arsenis e t ul., 1970). The fate of nucleosides arising within lysosomes in this way is not known. One possibility is the penetration ...
MB ChB PHASE I
... becomes the C of pyruvate and oxaloacetate respectively [as we saw in Lecture 1]. For other amino-acids, the conversion involves several steps. ...
... becomes the C of pyruvate and oxaloacetate respectively [as we saw in Lecture 1]. For other amino-acids, the conversion involves several steps. ...
Positional cues for the starch/lipid balance in maize kernels and
... combined topographical analysis (O2- and ATP-mapping), metabolite profiling, and isotope flux analysis. Internal and external oxygen levels were also experimentally altered. Under ambient conditions, mean O2 concentration immediately inside starchy endosperm dropped to only 1.4% of atmospheric satur ...
... combined topographical analysis (O2- and ATP-mapping), metabolite profiling, and isotope flux analysis. Internal and external oxygen levels were also experimentally altered. Under ambient conditions, mean O2 concentration immediately inside starchy endosperm dropped to only 1.4% of atmospheric satur ...
The Effect of Oxygen on the Growth and Mannitol
... Preparation of extracts. The organisms, grown to late-exponential phase, were harvested by centrifugation (21 OOOg, 10 min) at 4 "C and were subjected to the following procedures, which were carried out under strictly anaerobic conditions. The bacteria were washed three times with 50 mM-potassium ph ...
... Preparation of extracts. The organisms, grown to late-exponential phase, were harvested by centrifugation (21 OOOg, 10 min) at 4 "C and were subjected to the following procedures, which were carried out under strictly anaerobic conditions. The bacteria were washed three times with 50 mM-potassium ph ...
Bio 102 - Exam 2 Review 1
... Small, nonpolar, hydrophobic molecules such as fatty acids A. easily pass through a membrane's lipid bilayer. B. very slowly diffuse through a membrane's lipid bilayer. C. require transport proteins to pass through a membrane's lipid bilayer. D. are actively transported across cell membranes. BACK T ...
... Small, nonpolar, hydrophobic molecules such as fatty acids A. easily pass through a membrane's lipid bilayer. B. very slowly diffuse through a membrane's lipid bilayer. C. require transport proteins to pass through a membrane's lipid bilayer. D. are actively transported across cell membranes. BACK T ...
... utilizes hydrogen peroxide (H2O2) to catalyze hydroxylation and epoxidation at high rates. The cytochromes P450 are heme-containing oxygenases which collectively catalyze a variety of oxidations on a diverse array of substrates.[3, 8] The natural P450 catalytic cycle utilizes two electrons from NAD( ...
11. PHOTOSYNTHETIC PATHWAYS - Development of e
... It is the alternate pathway of C3 cycle to fix CO2. In this cycle, the first formed stable compound is a 4 carbon compound viz., oxaloacetic acid. Hence it is called C4 cycle. The path way is also called as Hatch and Slack as they worked out the pathway in 1966 and it is also called as C4 dicarboxyl ...
... It is the alternate pathway of C3 cycle to fix CO2. In this cycle, the first formed stable compound is a 4 carbon compound viz., oxaloacetic acid. Hence it is called C4 cycle. The path way is also called as Hatch and Slack as they worked out the pathway in 1966 and it is also called as C4 dicarboxyl ...
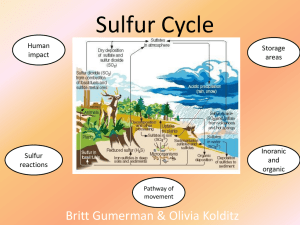
Sulfur Cycle - Walshearthsciences
... Sulfur reacts with fluorine, F2, and burns to form the hexafluoride sulfur(VI) fluoride. S8(s) + 24F2(g) → 8SF6(l) [orange] Reaction of sulfur with acids Sulfur does not react with dilute non-oxidizing acids. Reaction of sulfur with bases Sulfur reacts with hot aqueous potassium hydroxide, KOH, to f ...
... Sulfur reacts with fluorine, F2, and burns to form the hexafluoride sulfur(VI) fluoride. S8(s) + 24F2(g) → 8SF6(l) [orange] Reaction of sulfur with acids Sulfur does not react with dilute non-oxidizing acids. Reaction of sulfur with bases Sulfur reacts with hot aqueous potassium hydroxide, KOH, to f ...
Anaerobic and aerobic pathways for salvage of proximal tubules
... which is a product of both pathways A and B (Fig. 1), donates electrons to complex III via complex II (succinate dehydrogenase), bypassing the limitation of metabolism of complex I substrates (pathway C in Fig. 1). In this fashion, succinate-dependent aerobic respiration via normal electron transpor ...
... which is a product of both pathways A and B (Fig. 1), donates electrons to complex III via complex II (succinate dehydrogenase), bypassing the limitation of metabolism of complex I substrates (pathway C in Fig. 1). In this fashion, succinate-dependent aerobic respiration via normal electron transpor ...
Correlation of Hyperglycemia and Succinate dehydrogenase Activity
... The ultimate monosaccharide product of carbohydrate, glucose, is metabolized to yield energy required for the body. Glucose, after a series of reactions through glycolysis to produce pyruvic acid, subsequently enters Kreb’s cycle generating reduced nucleotides (NADH and FAD) for generating ATP, thro ...
... The ultimate monosaccharide product of carbohydrate, glucose, is metabolized to yield energy required for the body. Glucose, after a series of reactions through glycolysis to produce pyruvic acid, subsequently enters Kreb’s cycle generating reduced nucleotides (NADH and FAD) for generating ATP, thro ...
Structural Biochemistry/Enzyme/Active Site
... An active site is the part of an enzyme that directly binds to a substrate and carries a reaction. It contains catalytic groups which are amino acids that promote formation and degradation of bonds. By forming and breaking these bonds, enzyme and substrate interaction promotes the formation of the t ...
... An active site is the part of an enzyme that directly binds to a substrate and carries a reaction. It contains catalytic groups which are amino acids that promote formation and degradation of bonds. By forming and breaking these bonds, enzyme and substrate interaction promotes the formation of the t ...
... for the binding of the ligand and the reaction that is to be catalyzed. By studying the three-dimensional structure of a protein, it may be possible to identify possible binding sites through features that often are common for these. An active site may also be identified by introducing mutations in ...
Principles of BIOCHEMISTRY - Illinois State University
... • ATP is a substrate and an allosteric inhibitor of PFK-1 • High concentrations of ADP and AMP allosterically activate PFK-1 by relieving the ATP inhibition. • Elevated levels of citrate (indicate ample substrates for citric acid cycle) also inhibit Phospofructokinase-1 ...
... • ATP is a substrate and an allosteric inhibitor of PFK-1 • High concentrations of ADP and AMP allosterically activate PFK-1 by relieving the ATP inhibition. • Elevated levels of citrate (indicate ample substrates for citric acid cycle) also inhibit Phospofructokinase-1 ...
Biochemical Thermodynamics
... in the isolate system remains constant; energy may change form or it may be transported between regions (open subsystems constituting the total isolate system), but it cannot be created or destroyed (because of isolate system). The second 2nd law of thermodynamics, which can be stated in several for ...
... in the isolate system remains constant; energy may change form or it may be transported between regions (open subsystems constituting the total isolate system), but it cannot be created or destroyed (because of isolate system). The second 2nd law of thermodynamics, which can be stated in several for ...
SELECTIVE INHIBITORS OF DIHYDROFOLATE REDUCTASE
... laboratories for expansion of antibacterial and antimalarial testing. The antimalarial testing was included through the insight of Peter B. Russell, also a member of our research group. Russell noted the resemblance of a 5phenyl-2,4-diaminopyrimidine to a hypothetical conformation of the antimalaria ...
... laboratories for expansion of antibacterial and antimalarial testing. The antimalarial testing was included through the insight of Peter B. Russell, also a member of our research group. Russell noted the resemblance of a 5phenyl-2,4-diaminopyrimidine to a hypothetical conformation of the antimalaria ...
1.1 Functional Groups of Biomolecules and their Reactions
... definition. A Lewis acid is a molecule that accepts a pair of electrons and a Lewis base is a molecule that donates a pair of electrons. To accept electrons, a Lewis acid must have a vacant low-energy orbital. As a consequence, many species, including H+ itself, metal cations such as Mg2+ and Zn2+, ...
... definition. A Lewis acid is a molecule that accepts a pair of electrons and a Lewis base is a molecule that donates a pair of electrons. To accept electrons, a Lewis acid must have a vacant low-energy orbital. As a consequence, many species, including H+ itself, metal cations such as Mg2+ and Zn2+, ...
Special aspects of renal metabolism
... Urea is the major disposal form of amino groups derived from amino acids. Urea accounts for 90% of the nitrogen-containing components of urine. One nitrogen of the urea molecule is supplied by free NH3, and the other nitrogen by aspartate ...
... Urea is the major disposal form of amino groups derived from amino acids. Urea accounts for 90% of the nitrogen-containing components of urine. One nitrogen of the urea molecule is supplied by free NH3, and the other nitrogen by aspartate ...
Amino Acids: Disposal of Nitrogen & Urea Cycle
... OXIDATIVE DEAMINATION BY GLUTAMATE DEHYDROGENASE In mammals: almost entirely confined to the liver mitochondrial matrix / Readily reversible ...
... OXIDATIVE DEAMINATION BY GLUTAMATE DEHYDROGENASE In mammals: almost entirely confined to the liver mitochondrial matrix / Readily reversible ...
Amino Acids - faculty at Chemeketa
... Solution Match the end products of digestion with the types of food: 1. amino acids 2. fatty acids and glycerol 3. glucose A. fats B. proteins C. carbohydrates ...
... Solution Match the end products of digestion with the types of food: 1. amino acids 2. fatty acids and glycerol 3. glucose A. fats B. proteins C. carbohydrates ...
COX-1 And COX-2 Enzymes Synthesize Prostaglandins and Are
... acid, a fatty acid in cell membranes, into prostaglandins, modified fatty acids attached to a ring of five carbons. COX stands for cyclooxygenase meaning that it is an enzyme that oxidizes a substrate. The prostaglandins that the two enzymes produce are identical, but get converted into different pa ...
... acid, a fatty acid in cell membranes, into prostaglandins, modified fatty acids attached to a ring of five carbons. COX stands for cyclooxygenase meaning that it is an enzyme that oxidizes a substrate. The prostaglandins that the two enzymes produce are identical, but get converted into different pa ...
Oxygen diffusion through perovskite membranes
... determined by a thermogravimetric analyzer (TA Instruments). The membrane tube was prepared by the plastic extrusion method [19]. The sintered membrane tube has an outer diameter of about 8.0 mm, an inner diameter of about 5.0 mm. The densities of the sintered tubular membranes were measured by the ...
... determined by a thermogravimetric analyzer (TA Instruments). The membrane tube was prepared by the plastic extrusion method [19]. The sintered membrane tube has an outer diameter of about 8.0 mm, an inner diameter of about 5.0 mm. The densities of the sintered tubular membranes were measured by the ...
Oxidative phosphorylation
Oxidative phosphorylation (or OXPHOS in short) is the metabolic pathway in which the mitochondria in cells use their structure, enzymes, and energy released by the oxidation of nutrients to reform ATP. Although the many forms of life on earth use a range of different nutrients, ATP is the molecule that supplies energy to metabolism. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is probably so pervasive because it is a highly efficient way of releasing energy, compared to alternative fermentation processes such as anaerobic glycolysis.During oxidative phosphorylation, electrons are transferred from electron donors to electron acceptors such as oxygen, in redox reactions. These redox reactions release energy, which is used to form ATP. In eukaryotes, these redox reactions are carried out by a series of protein complexes within the inner membrane of the cell's mitochondria, whereas, in prokaryotes, these proteins are located in the cells' intermembrane space. These linked sets of proteins are called electron transport chains. In eukaryotes, five main protein complexes are involved, whereas in prokaryotes many different enzymes are present, using a variety of electron donors and acceptors.The energy released by electrons flowing through this electron transport chain is used to transport protons across the inner mitochondrial membrane, in a process called electron transport. This generates potential energy in the form of a pH gradient and an electrical potential across this membrane. This store of energy is tapped by allowing protons to flow back across the membrane and down this gradient, through a large enzyme called ATP synthase; this process is known as chemiosmosis. This enzyme uses this energy to generate ATP from adenosine diphosphate (ADP), in a phosphorylation reaction. This reaction is driven by the proton flow, which forces the rotation of a part of the enzyme; the ATP synthase is a rotary mechanical motor.Although oxidative phosphorylation is a vital part of metabolism, it produces reactive oxygen species such as superoxide and hydrogen peroxide, which lead to propagation of free radicals, damaging cells and contributing to disease and, possibly, aging (senescence). The enzymes carrying out this metabolic pathway are also the target of many drugs and poisons that inhibit their activities.