* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Positional cues for the starch/lipid balance in maize kernels and
Survey
Document related concepts
Plant nutrition wikipedia , lookup
Microbial metabolism wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Photosynthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Plant breeding wikipedia , lookup
Cryobiology wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Citric acid cycle wikipedia , lookup
Embryo transfer wikipedia , lookup
Biochemistry wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Transcript
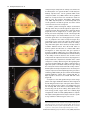
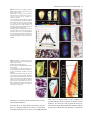
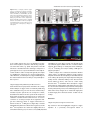
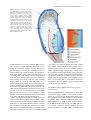
The Plant Journal (2005) 42, 69–83 doi: 10.1111/j.1365-313X.2005.02352.x Positional cues for the starch/lipid balance in maize kernels and resource partitioning to the embryo Hardy Rolletschek1, Karen Koch2, Ulrich Wobus1 and Ljudmilla Borisjuk1,* Institut für Pflanzengenetik und Kulturpflanzenforschung (IPK), Corrensstr. 3, 06466 Gatersleben, Germany, and 2 Plant Molecular and Cellular Biology Program, Horticultural Sciences Department, University of Florida, 1143 Fifield Hall, PO Box 110690, Gainesville, FL 32611, USA 1 Received 22 October 2004; revised 17 December 2004; accepted 23 December 2004. * For correspondence (fax þ49 39482 5500; e-mail [email protected]). Summary This study tests the hypotheses that in vivo oxygen levels inside developing maize grains locally affect assimilate partitioning and ATP distribution within the kernel. These questions were addressed through combined topographical analysis (O2- and ATP-mapping), metabolite profiling, and isotope flux analysis. Internal and external oxygen levels were also experimentally altered. Under ambient conditions, mean O2 concentration immediately inside starchy endosperm dropped to only 1.4% of atmospheric saturation (approximately 3.8 lM), but was 10-fold higher in the oil-storing embryo. Increasing the O2 supply to intact kernels stimulated their O2 demand, shifted ATP localization within the kernel, and elevated their ATP/ADP ratio. Enhanced O2 availability also increased steady-state levels of glycolytic intermediates and those of the citric acid cycle, as well as some related pools of free amino acids. Subsequent analyses indicated that starch formation within endosperm, but not lipid biosynthesis within embryo, was adapted to the endogenous low oxygen. Increasing the O2 supply did not change ADP-glucose levels, activity of ADP-glucose pyrophosphorylase, 13C-labeling of ADP-glucose, or flux of 14C-sucrose into starch. In contrast, enhanced O2 availability increased 14C-label uptake into the embryo, 13C-labeling of acetyl-coenzyme A, and finally 14C-incorporation into lipids. Lipid accumulation in embryo appeared highest in regions with higher ATP. Consistent with labeling data, a decrease in O2 supply most strongly affected the embryo, whereas rising O2 levels expanded ATP-rich zones toward the starch-storing endosperm and the scutellar part of embryo. The latter might be responsible for higher 14C-label uptake into the embryo and flux toward lipid. Collectively, data indicate that the in vivo oxygen distribution in maize kernels markedly affects ATP gradients, metabolite levels, and favors assimilate partitioning toward starch within the O2-depleted endosperm. Clear advantages are thus evident for peripheral localization of the protein and lipid storing structures in maize kernels. Keywords: lipid and starch metabolism, metabolite imaging, seed development, assimilate partitioning, energy state, hypoxia. Introduction Maize is one of the world’s top three grain crops (Chrispeels and Sadava, 2003), with starch, lipid, and protein deposition in this kernel accounting for a significant portion of the global food supply. Its endosperm is the major site of starch storage, whereas lipid biosynthesis is favored in its embryo (80 and 30%, respectively; Doehlert, 1990). Extensive study of assimilate partitioning between these seed structures and storage components has shown their regulation to involve a complex interplay of gene expression and metabolism during development (Bate et al., 2004; Becraft, 2001; Choi et al., ª 2005 Blackwell Publishing Ltd 1997; Focks and Benning, 1998; Hannah and Greene, 1998; Lin et al., 1999; Sakulsingharoj et al., 2004; Wobus and Weber, 1999). Kernel development is supported by sucrose transferred from phloem, across pedicel and basal transfer cells (Aoki et al., 1999; Thomas et al., 1992) to the main endosperm compartments and embryo (Shannon, 1972). Sucrose is partly cleaved and re-synthesized within the endosperm (Cheng, 1997), which in turn might affect developmental state (Wobus et al., 2004) and expression of storage-associated 69 70 Hardy Rolletschek et al. genes (Koch, 1996, 2004). Sugars subsequently move toward the upper endosperm where starch storage is initiated. Flux toward the embryo seems to be tightly controlled by specific features of the embryo-surrounding tissue, responsible mechanisms being largely unknown, but probably involving invertases and their inhibitors (Bate et al., 2004). Starch biosynthesis in endosperm often correlates with activity of sucrose synthase (Doehlert, 1990; Winter and Huber, 2000) and is typically limited by ADP-glucose pyrophosphorylase activity (Hannah and Greene, 1998; Salamone et al., 2002). Lipid biosynthesis in the embryo seems more closely associated with invertase and hexokinase activity (Doehlert, 1990). Enzymes of glycolysis, citrate cycle, starch, protein and lipid biosynthesis are reported to be coordinately expressed in both endosperm and embryo (Giroux et al., 1994; Lee et al., 2002). Their activity determines local sink strength which in turn may regulate assimilate partitioning into different storage product classes. Recent data suggest that low internal oxygen concentrations may also play a pivotal role in assimilate partitioning. Oxygen depletion appears to be a common feature of many plant tissues, including developing seeds and grains (Rolletschek et al., 2002a; Shelp et al., 1995; Vigeolas et al., 2003). Endogenous hypoxia was shown to decrease assimilate import and storage metabolism by wheat grains (VanDongen et al., 2004), and to alter energy state of growing barley kernels (Rolletschek et al., 2004a). Analysis of diverse dicot seeds also showed that endogenous oxygen limitation affected resource allocation among storage products (Borisjuk et al., 2003; Rolletschek et al., 2003). In embryos of the dicot, oilseed rape, Vigeolas et al. (2003) could show that lipid but not starch storage metabolism was limited by the prevailing low oxygen levels. Photosynthesis was also shown to provide O2 to hypoxic seed tissues (Rolletschek et al., 2004a) as well as energy (ATP/NADPH; Ruuska et al., 2004). There are several reasons why developing maize kernels may be subject to still greater effects of low oxygen than the dicot seeds and smaller grains. One is that the surface/volume ratio of the large maize kernels would be smaller. In addition, maize kernels are distinct in (i) their virtually complete lack of photosynthetic activity in the grain, and (ii) the extensive non-vascular region (no lateral vein supplies the grain). These factors indicate that endogenous oxygen deprivation may be particularly pronounced inside developing maize kernels, and that evolution of this largest cereal grain may have involved special adaptations to oxygen limitation. Significant implications of oxygen depletion (in sensu energy limitation) could include altered input into signaling systems, gene expression, and/or metabolic control (Geigenberger, 2003; Greenway and Gibbs, 2003; Koch et al., 2000; Subbaiah and Sachs, 2001). Both transport and phosphorylation of hexoses via hexokinases are affected by low O2/ATP (Bouny and Saglio, 1996; Geigenberger, 2003; Trethewey et al., 1999; Xia and Saglio, 1990). In addition, maize sucrose synthases (Sus1 and Sh1) respond differentially to both sucrose and low oxygen (Zeng et al., 1998). Moreover, the balance between activity of the sucrose-cleaving enzymes, invertase and sucrose synthase, is affected by low O2 (Zeng et al., 1999). This in turn would be expected to alter the hexose/sucrose state of the cells, and partitioning of C-flux between storage and respiration. The overall motivation for this work was to investigate the extent of potential oxygen depletion in functionally different regions of maize kernels, and determine the degree to which this limits key aspects of kernel metabolism: assimilate partitioning and C-flow to the embryo. Toward this end, we combined topographical analysis of ATP gradients across tissues in developing maize kernels, with microsensor quantifications of O2 in transects through kernels, and assays of energy balance, metabolite profiles, and labeling studies with 13C- and 14C-assimilates. Each approach was applied to experimental perturbations of oxygen inside intact, attached kernels. Results show a marked degree of oxygen deficiency inside developing maize kernels. Moreover, data reveal that young kernels contend with significant oxygen limitation to ATP gradients, energy status, metabolite pools, assimilate delivery to developing embryos, and partitioning of sucrose to non-starch storage compounds. Starch storage in endosperm, however, appears specifically adapted to the localized low-oxygen environment inside these, and possibly other grains. Results Oxygen is severely depleted inside developing maize kernels Experiments were performed during the near-linear phase of starch deposition in developing maize kernels (from about 100 to 350 mg fresh weight, approximately 12–42 days after pollination, DAP). Onset of this stage was marked by increased levels of ADP-glucose (the direct precursor for starch synthesis) (Figure 1a). Amounts of glycolytic intermediates did not change significantly during this period, but free amino acids generally declined (data not shown). The ratio of whole-kernel ATP to ADP remained relatively constant, but decreased somewhat during the storage phase (Figure 1b), consistent with overall increases in ATP demand versus ATP supply. A 10-fold decrease in the kernel hexose/ sucrose ratio was observed at the onset of starch storage (9.8 versus 0.8). However, hexose levels were considerably lower in embryos than in the (mainly) starch-storing endosperm (Figure 1c), indicating a distinctly different metabolic status. Such depletion of hexoses relative to sucrose in embryos could readily alter sugar signals in these structures, ª Blackwell Publishing Ltd, The Plant Journal, (2005), 42, 69–83 Positional cues for resource partitioning 71 larger kernels. In contrast to endosperm, embryos showed two remarkable features: (i) Mean O2 levels remained considerably higher (at about 13.5 6.5%). (ii) Concentration gradients descended from relatively high levels at the outer surface of the embryo (10–20%) to barely detectable amounts at the embryo/endosperm interface (innermost scutellar surface). This concentration gradient suggests that diffusive influx through the embryo may be an important source of oxygen for the scutellum and the embryosurrounding region. ATP is gradiently distributed within the kernel tissues Figure 1. Biochemical parameters of developing maize kernels. (a) Dynamic of starch accumulation (mg per kernel) and ADP-glucose levels (nmol g)1 fresh weight). (b) Decrease of ATP/ADP-ratio during starch storage. (c) Contrast between mean sugar status of embryo and endosperm (25 DAP). Error bars are mean standard deviation. and thus differentially affect developmental programs (Bate et al., 2004; Wobus and Weber, 1999). Internal oxygen levels were quantified in young kernels using microsensors with tip diameters of approximately 50 lm, which allowed a high degree of spatial resolution (Figure 2). The O2 level dropped dramatically within the first 500 lm of the kernel surface. The remainder of the endosperm showed little detectable O2, with mean levels in the interior reaching only 1.4 1.0% saturation (atmospheric O2 level of approximately 21 kPa is set to 100%). Minimum levels dropped below resolution of the microsensor (<0.1%). As the microsensor tip moved through the kernel and approached the pericarp surface on the opposite side, O2 levels again increased steeply and nearly symmetrically. The mean O2 levels within the endosperm were equally depressed in kernels from 12 to 45 DAP, but were considerably higher in kernels prior to onset of starch storage (<10 DAP; data not shown). Measurements of O2 levels within the embryo alone were technically not feasible for kernels smaller than 200 mg, but were reproducible for ª Blackwell Publishing Ltd, The Plant Journal, (2005), 42, 69–83 We further analyzed spatial ATP distribution and whether gradients in ATP are related to those in local oxygen levels and storage-product deposition. ATP distribution was determined directly in cryosections of snap-frozen kernels via bioluminescence imaging (Borisjuk et al., 2003). Data from whole kernels sectioned as in Figure 3(a), and shown in Figure 3(b,d,e), reveal that ATP levels were low in peripheral regions of kernels (pericarp, pedicel, aleurone, and outermost endosperm). ATP levels remained high throughout most of the inner endosperm, with steep concentration gradients at the periphery (Figure 3c). ATP content increased almost linearly from peripheral endosperm regions with small cells toward inner regions with larger cells. More gradual ATP gradients were evident between basal and apical parts of the endosperm. Transverse sections along the central a-axis (Figure 3d) also showed that ATP levels were low in peripheral regions of the kernel, but higher in conducting tissues. Greatest ATP levels (up to 1000 nmol g)1 FW) were observed in the endosperm region immediately adjacent to the embryo base. ATP concentration dropped markedly in the lower endosperm, particularly in the gap region, the transfer cells, and in adjacent tissues. Content of ATP was lower in the embryo axis than in the endosperm, but in scutellum, an ATP gradient extended from the abaxial epidermis toward the inner zone, peaking in the abaxial nodular region. Scutellar procambium and inner tissues of the embryo (coleoptile, plumule, primary root and others) showed low ATP levels. Within maternal tissues (shown along the c-axis in Figure 3e), ATP levels were slightly elevated in the spongy parenchyma, but very low in the vascular bundle. ATP content was also low at the endosperm/pericarp interface. A comparison of Figure 3(c,f) shows that ATP levels were generally higher in large cells of the interior endosperm and embryo-surrounding regions than in more dense tissues with small cells. For each region of ATP analysis (Figure 3c), tissue masks in gray scales (base of Figure 3c) delineated distribution of small cells in peripheral endosperm and larger cells in loose tissues of the central region (as pictured in Figure 3f). The layers of endosperm cells lining the embryo pocket also contained larger cells than the outer 72 Hardy Rolletschek et al. endosperm layers away from the embryo (not shown). The described pattern is in agreement with a model that quantitatively reproduced the cell-size gradient within maize endosperm (Vilhar et al., 2002). Similar cell-size gradients within the endosperm have been described in detail for different maize lines (Kowles and Phillips, 1988; Larkins et al., 2001). Superimposition of our ATP maps with such cell-size gradients revealed the general association of ATP content and cell size in the endosperm. In addition, parallel histological analysis showed that although starch was present throughout the upper half of endosperm (Figure 4a,b), gradients were evident in density (Figure 4b) and size (Figure 4c,d) of starch grains. The density decreased basipetally in both central (Figure 4b) and lateral regions (not shown). Differences in size of cells and starch grains were most clearly apparent in comparisons of peripheral and central endosperm (Figure 4c,d, respectively). Large central cells held the biggest starch grains (with lowest surface-to-volume ratios). In embryo, only small starch grains were observed, mostly located in scutellum. Maternal tissues were filled with starch as well. The pattern described here is consistent with earlier investigations of starch deposition and gene expression (Perdomo and Burris, 1998; Young et al., 1997). Lipid staining with Sudan B red showed that lipids accumulated mainly in the embryo and in the aleurone cells as well as in the endospermal cells adjacent to the aleurone layer (Figure 4e). Only residual levels of lipids were detectable in the central endosperm or embryo-attached regions. Strong lipid gradients were visualized within the embryo (stained image in Figure 4f). Lipids accumulated mainly in the region facing the endosperm and decreased toward the embryo interior. Similar spatial and temporal patterns were observed previously for expression of the L3 lipid body protein gene (Vance and Huang, 1988). Notably, the present work shows that lipid gradients within the embryo were coupled with ATP, i.e. lipid deposition decreased concomitant with ATP levels (Figure 4f). A comparison of the ATP gradient maps to both storage pattern and oxygen distribution within the kernel supported the following observations: (i) In endosperm, where starch is the main storage product, oxygen concentration was very low and high ATP levels were associated with enlarged, starch-storing cells. (ii) In the embryo, where lipids are the main storage product, oxygen levels were relatively high and gradients of ATP coincided spatially with those of lipids. This implies that high ATP levels may be related to specific storage activities in different regions of the seed. Figure 2. Representative oxygen maps within developing maize kernels. O2 was measured by microsensors along the x-axis (penetration depth given in lm) within kernels at (a) 130 mg fresh weight (15 DAP), (b) 230 mg fresh weight (25 DAP), and (c) 300 mg fresh weight (35 DAP). The O2 concentration is given in % of atmospheric saturation (approximately 21 kPa ¼ 100%). ª Blackwell Publishing Ltd, The Plant Journal, (2005), 42, 69–83 Positional cues for resource partitioning 73 Figure 3. Bioluminescence imaging of ATP distribution within maize kernels during the peak period of starch storage. (a) Orientation of a, b, and v sections through the maize kernel. ATP concentrations are given in a color scale at the base (en, endosperm; e, embryo; p, pericarp). (b, d, e) Left panels represent ATP maps (gradients and their physical localization) measured directly within cryosections positioned as shown for a, b, and v sections in the right panels (BE, basal endosperm; CE, central endosperm; P, pericarp; PC, pedicel; PE, peripheral endosperm; Sc, scutellum; VB, vascular bundle). (c) Graph of ATP concentration measured across the dashed arrow in the right panel of (b) is pictured in relation to (f) a co-localized gradient in cell size with iodine staining of amyloplasts in this starchy endosperm tissue. Cell conture is marked by the white line to visualize the cell-size gradient. Figure 4. Distribution of storage products in longitudinal sections of kernels during the peak period of starch storage. (a) Hand-section through the kernel. (b) Starch deposition visualized in dark-brown by iodine staining. (c, d) Comparison of starchy endosperm cells from peripheral (c) and central (d) endosperm. (e) Lipid accumulation visualized in red by Sudan B. (f) Lipid deposition within embryo in relation to concentration gradients for ATP (line graph) and oxygen (circles). Both ATP and O2 levels were measured along a transect line (white arrow) also shown as the x-axis in panel (f). The ATP line graph represents a polynomial regression curve fitted to individual ATP analyses (black dots) (Al, aleurone; G, germ; Pc, pedicel; e, embryo; en, endosperm; Sc, scutellum). External O2 concentration affects internal O2 levels, energy state and ATP distribution As shown above, O2 levels within kernels fall to very low levels. To determine whether the interior of developing ª Blackwell Publishing Ltd, The Plant Journal, (2005), 42, 69–83 maize seeds are hypoxic under in vivo conditions, we experimentally altered the exogenous O2 supply to kernels (Figure 5). The microsensor was inserted into intact, attached kernels (about 350 lm depth), followed by a stepwise change of the ambient O2 levels. Reduction of external O2 74 Hardy Rolletschek et al. (Figure 6a,b). The increase in energy levels resulting from a rise in oxygen levels from the 10 to 200% level was more marked for older than younger kernels. To see if changes in the ATP level affected the whole kernel or (even more importantly) were confined to distinct regions otherwise concealed by whole organ analysis, we investigated the ATP distribution using bioluminescence imaging. When kernels were exposed to 10% O2, ATP concentration decreased most markedly in the basal and middle endosperm region near the embryo (Figure 6c). However, when exogenous O2 was raised to 200% ATP-rich regions began expanding into upper parts of the endosperm, the embryo surrounding region and the embryo itself. In later stages of kernel growth (Figure 6d), the decrease in ATP at the 10%-level, as well as the increase at the 200%-level, was primarily associated with the embryo and the embryo-surrounding region. These results imply that an increase in O2 supply leads to higher energy levels and altered ATP distribution patterns. Data also indicate that ATP production in developing kernels is limited in vivo by low internal oxygen levels (hypoxia). Oxygen availability alters steady-state levels of metabolites and enzyme activities Figure 5. Effect of elevations in external oxygen levels on internal O2 concentration in the central endosperm of the maize kernel (25 DAP). The level of O2 is given in % of atmospheric saturation (approximately 21 kPa ¼ 100%; mean standard deviation). availability drove internal concentrations below levels of detection within 3–5 min. Increases in external O2 concentration, up to approximately 200% (representing 42 vol.% ¼ twofold atmospheric O2 saturation), did not affect the internal level (Figure 5). More marked elevations of external O2 concentration eventually raised endosperm levels above those normally observed in vivo. Rising O2 supply was thus apparently balanced by increasing O2 consumption. External levels greater than 200% were needed before internal O2 demand could be exceeded. Consequently, respiration inside developing kernels was considered to be non-O2-saturated at normal ambient levels, i.e. was O2-limited in vivo. To test if changes in respiratory O2 demand induced by varying external O2 supply resulted in elevated energy levels, intact kernels were treated with gas mixtures containing either 10, 100, or 200% of the ambient O2 level (corresponding to 2.1, 21, and 42 vol.% of O2, respectively). The concentration of adenine nucleotides in whole kernel extracts increased progressively with increasing external O2 levels (Figure 6a,b). The ATP/ADP ratio also increased significantly in response to elevated external O2 Differential responses to changes in O2 supplies are shown in Figure 7 (see also supplementary Materials Table S1) for intermediates of glycolysis and the citric acid cycle, as well as for related sugars and free amino acids. The positioning of sub-figures is based on their proximity in a generalized metabolic context (for details see Dwyer et al., 2004). Although soluble sugar levels did not change in response to shifts in external O2 supply, UDP-glucose content dropped markedly when oxygen availability was doubled. Sucrose synthase activity also declined from 2.8 (0.2) to 2.0 (0.4) lmol g)1 FW min)1. In contrast, no significant change was observed for either the ADP-glucose pool size or activity of ADP-glucose pyrophosphorylase [2.4 (0.3) lmol g)1 FW min)1]. Levels of most glycolytic intermediates rose progressively with increasing O2 supply, but those of pyruvate were maximal when exogenous O2 supplies (10%) were lowest. The lack of accompanying increases in either lactate or alanine, indicated an absence of lactate/alanine fermentation even under these low-O2 conditions. Metabolite levels that increased most markedly in response to superambient oxygen were Fruc1,6diP and acetyl-coenzyme A, the latter being a precursor for plastidic fatty acid synthesis as well as mitochondrial citrate cycle, amino acid synthesis, etc. Within the pool of organic acids, only citrate and isocitrate showed significant, progressive increases with rising O2 availability, and similar patterns were evident for Glu, Asp, Ser, and some of the other free amino acids [Gln, Gly, Pro (not shown)]. Together, observed changes showed that increases in O2 levels allowed supplies of glycolytic intermediates ª Blackwell Publishing Ltd, The Plant Journal, (2005), 42, 69–83 Positional cues for resource partitioning 75 Figure 6. Effect of changing external oxygen levels on concentration of adenine nucleotides, ATP/ADP ratio and ATP distribution in maize kernels at 27 DAP (a, c) and 42 DAP (b, d). Intact kernels were aerated with gas mixtures containing 10, 100 and 200% of ambient oxygen levels (black, gray, and white columns, respectively). Data in (a) and (b) are given as mean standard deviation. Concentration of ATP in (c) longitudinal and (d) transverse sections is given in a color scale on the base of the figure (e, embryo; en, endosperm). to rise relative to their rates of use as substrates for respiration and biosynthesis (collective data favoring end-products other than starch, e.g. lipids and proteins). This will include the observation that the starch-type endosperm of grains appears to be a special modification not broadly observed elsewhere. Other endosperms typically store lipids (e.g. Arabidopsis, castor bean, etc.) and are peripherally located. However, seed development, oxygen availability, and the relation between them are likely to vary markedly between species. Oxygen supply shifts partitioning of labeled sucrose between different storage products and C-flux to the embryo Further analysis of oxygen effects on metabolic partitioning were undertaken using 13C- and 14C-sucrose delivered by a microliter injection system into the endosperm of intact, attached kernels. Data from mass spectrometric analyses in Figure 8(a,b) show that a twofold increase in external O2 resulted in a similar twofold enhancement of 13C-sucrose conversion to 13C-acetyl-coenzyme A. No significant changes were evident for effects of oxygen enhancement on transfer of label to 13C-ADP-glucose (Figure 8b), consistent with metabolite patterns described above. Figure 8(c) shows that 14C-incorporation into starch was slightly reduced at ª Blackwell Publishing Ltd, The Plant Journal, (2005), 42, 69–83 subambient O2 levels (10%), but was not affected by the superambient supplies (200%). This line of evidence further supports above findings on steady-state levels of ADP-glucose, its 13C-labeling, and enzyme activity of ADP-glucose pyrophosphorylase. Further analyses were conducted to determine whether oxygen effects on partitioning between storage products may have involved an altered capacity for assimilate transfer to the embryo (the primary site of lipid deposition being the embryo and its scutellum). Embryos were dissected from kernels labeled as above, and found to contain nearly threefold more 14C-assimilates when atmospheric oxygen levels had been doubled (Figure 8d). Similar increases were also observed for total label incorporated into embryo lipids and the pellet (starch, protein, and cell wall) (Figure 8e). On a whole kernel level, changes due to elevated O2 supply thus resulted largely from enhanced transfer of label into the embryo. Discussion Oxygen is depleted to hypoxic levels in vivo Key evidence in this work highlights endogenous oxygen depletion as a previously unrecognized feature of 76 Hardy Rolletschek et al. Figure 7. A simplified schematic representation of primary metabolites in maize kernels (25 DAP) and their response to changing O2 supply. Intermediates of glycolysis and the citrate cycle are shown, as well as branch paths to related sugars and free amino acids. Arrows indicate probable direction of predominant C flow. Vertical bars show the level of each metabolite in kernels after a 6-h treatment with 10, 100 and 200% ambient O2 level (black, dark gray and gray bars, respectively). Data are given in relative units (mean standard deviation). developing maize kernels (Figures 1 and 2). The extent and duration of this hypoxia are marked. Storing kernels show strongest O2 gradients immediately inside the peripheral endosperm, declining to very low levels within the first 200–400 lm. This indicates a high O2 demand that outstrips the diffusive capacity of the kernel. Hypoxic conditions Figure 8. Fluxes to metabolic intermediates and storage products in response to changing O2 supply. (a, b) Amount of 13C-labeled acetyl-coenzyme A and ADP-glucose, respectively, after delivery of 13C-sucrose (3 ll, 200 mM) into the central endosperm by an air-sealed minute injection system, and incubation (2 h) of intact kernels (on ear) at ambient (100%) and superambient (200%) O2 levels. (c) Partitioning of 14C-sucrose into starch and the remaining pellet (mainly protein and cell wall) after air-sealed, minute injection of 3 ll 14C-sucrose into inner endosperm and incubation (6 h) at 10, 100 and 200% of ambient O2 level. (d, e) Uptake and incorporation of 14C-label into different fractions in maize embryos as affected by elevated external oxygen. Radiolabeled14C-sucrose (3 ll) was delivered via air-sealed minute injection into inner endosperm of kernels attached to the plant. After incubation (6 h) at ambient oxygen levels (100%) and superambient levels (200%), the embryo was isolated and analyzed. All data are mean standard error. Significant differences versus control treatment (100%) are given by *; t-test, P < 0.05). ª Blackwell Publishing Ltd, The Plant Journal, (2005), 42, 69–83 Positional cues for resource partitioning 77 Figure 9. Schematic representation of oxygen and sugar delivery in storing maize kernels. Oxygen enters the kernel via the entire surface (blue arrows), and diffuses along steep concentration gradients toward the interior. Oxygen becomes depleted within the endosperm, but maintains higher levels within the embryo, possibly indicating a superior O2 supply. Sugars (red arrows) enter the kernel via the pedicell/ transfer cell region and flow toward the starchsynthesizing crown area of endosperm and the lipid-synthesizing embryo via scutellum. High ATP regions in the endosperm are found adjacent to the embryo base and within starchstoring cells. Within embryo (see insert), high ATP and sugar supply overlap in regions where lipid accumulation occurs. prevail throughout most of the endosperm. Within the embryo, O2 levels are significantly higher. Distinct O2 levels in embryo and endosperm might be caused by differences in either O2 supply or respiratory O2 consumption. Lower respiratory activity of the embryo is barely to be expected due to the requirement of ATP for lipid and protein synthesis. A superior O2 supply to the embryo seems more likely. In dicot seeds, the region of seed coat attached to the embryo axis (root pertrusion zone) is the main site of gas exchange (Wager, 1974). O2 content in maize endosperm falls to very low levels (<0.1%), far below those observed in green seeds of any other species examined thus far (Vicia/Pisum: Rolletschek et al., 2002a, 2003; oilseed rape: Vigeolas et al., 2003). Only the chlorophyll-free endosperm of other cereals approach such low O2 levels (Rolletschek et al., 2004a). Maize kernels may thus be more susceptible to oxygen depletion and internal hypoxia than are many developing seeds and/or have developed specific adaptations. Maize kernels lack photosynthesis, unlike seeds and grains of many other crop plants. Oxygen supply to developing kernels thus depends entirely on diffusion from the ambient air, so is governed by diffusive resistance of tissues, O2 concentration gradients, and atmospheric O2 level. Oxygen profiles suggest that O2 uptake occurs via the entire ª Blackwell Publishing Ltd, The Plant Journal, (2005), 42, 69–83 seed surface (see Figure 9). Oxygen flow from pedicell layers toward endosperm might also be possible because of elevated O2 levels in these layers. Concentration gradients further suggest that oxygen flows from the embryo toward the endosperm, thereby oxygenating the embryo surrounding region. This may have an important regulatory function for assimilate uptake into the embryo as discussed below. Oxygen supply via phloem/xylem seems negligible because both mass flow rates and O2 concentrations within this liquid (VanDongen et al., 2003) are very low compared with overall respiratory O2 demand. ATP gradients reflect metabolic state of tissues, and are O2-responsive Data revealed markedly steep ATP-gradients in developing maize kernels (Figures 3 and 4). In endosperm, this distribution also corresponded to growth and maturation patterns. Cell divisions cease first in central endosperm (Kowles and Phillips, 1988), followed by endoreduplication of their DNA (Schweizer et al., 1995), and cell expansion (Larkins et al., 2001; Arabidopsis; Kondorosi et al., 2000). This process extends from the endosperm crown to the basal transfer cells (Larkins et al., 2001; Vilhar et al., 2002), and is 78 Hardy Rolletschek et al. closely associated with cellular differentiation (Nagl et al., 1985). Starch accumulation occurs as cells expand, whereas cell division continues only in peripheral cell layers, away from the embryo (Larkins et al., 2001). Finally, cell death is initiated in the central and upper endosperm, progressing toward the base and periphery of the kernel (Young et al., 1997). Along this developmental gradient, we found that low ATP was associated with developmentally younger tissues that maintain some mitotic activity and accumulate starch at low rates (Figures 3 and 4). ATP levels rose with the extent of cell differentiation, endoreduplication, and starch accumulation. Two apparent exceptions involved low ATP levels in mature cells, but the first of these were endopolyploid cells in the central endosperm that had begun to die, and the second were cells in basal regions specialized for solute transport, but not starch accumulation (BELT region with distinct genetic regulation, Hueros et al., 1999). Overall, topographical evidence showed that ATP concentration was apparently coupled to gradients in cell size and endoreduplication (Larkins et al., 2001), expression of ADP-glucose pyrophosphorylase (Brangeon et al., 1997), starch accumulation (Young et al., 1997), and associated demands for nucleotide precursors and metabolic activity (Baluka and Kubica, 1992). A similar relationship was previously identified for tissue gradients in ATP and local onset of starch storage in the barley caryopsis (Rolletschek et al., 2004a). Results of both studies support the suggestion that high local energy levels are indicatory for the metabolic status of the tissue, and may represent an important feature of expanding cells undergoing endopolyploidization and storing starch. The physiologic importance of this observation has to be further investigated. ATP gradients in the embryo axis and scutellum also corresponded with those of storage deposition, but primarily for lipids in this instance (Figures 3 and 4). No association was evident between this ATP distribution analyzed here and patterns of cell division (Jose-Estanyol et al., 1992; Stiefel et al., 1999), without endopolyploidization (Killian et al., 1998). Storage processes in the scutellum begin in the developmentally older basal regions and proceed toward the apex, first with starch deposition, and then lipids (Perdomo and Burris, 1998). A gradient in lipid accumulation was also evident with the maximum concentration nearest the endosperm interface (Figure 4f), and this correlated with ATP distribution. In contrast, oxygen gradients in the scutellum (Figure 4f) were inverse to those of lipids and ATP (Figure 4f), consistent with high rates of local consumption. Carbohydrates used for lipid biosynthesis move within the scutellum from the endosperm interface toward the interior cells (Griffith et al., 1987; Matthys-Rochon et al., 1998), and may therefore show gradients similar to those of lipids and ATP. It is tempting to speculate that zones favorably supplied with both carbohydrates (from the kernel interior) and oxygen (from the exterior), could yield the greatest levels of both ATP and lipids (see Figure 9). In addition to genetic control of lipid biosynthesis (Boyer and Hannah, 2001), local energy status may contribute prominently to its in vivo regulation. Oxygen availability limits energy status and ATP distribution in maize kernels A marked influence was demonstrated for normal degrees of endogenous oxygen depletion, by experimentally altering oxygen supplies to intact, attached kernels. Data in Figure 5 demonstrate that O2 demand of developing kernels is not saturated at ambient (atmospheric) O2 levels or even by a twofold increase in the exogenous supply. Enhanced O2 availability is coupled with higher ATP levels, increased ATP/ ADP ratio, and thus elevated overall energy state of tissues (Figure 6). Bioluminescence imaging also showed that increased O2 availability expanded distribution of ATP-rich zones from the kernel periphery and embryo into the starchstoring endosperm and tissues of the scutellar interior (Figure 6). A decrease in O2 supply affected the embryo most strongly (Figure 6c,d), presumably the result of its contrasting metabolic status (sugar composition, storage metabolism, etc.) relative to endosperm. Starch biosynthesis in endosperm is adapted to hypoxia, but lipid synthesis in embryo is limited by in vivo oxygen supply Further significance of work shown here lies in its demonstration that oxygen depletion inside normally growing maize kernels is extreme enough to significantly alter pools of metabolic intermediates (Figure 7) and their partitioning between storage end-products (Figure 8). Enhancement of oxygen available to storing kernels increased steady-state levels of glycolytic intermediates (hexose phosphates, Fruc1,6-diP, PEP, 3-PGA) and citric acid cycle metabolites (acetylcoenzyme A, citrate, isocitrate), as well as some related free amino acids (Figure 7). Supplies of these precursors and substrates thus rose relative to their rate of use in biosynthesis and respiration. Conversely, further reductions in oxygen availability [to 1/10 of normal ambient levels (Figures 6 and 7)], decreased the energy state (ATP/ADP ratio) and lowered levels of glycolytic intermediates (regardless of flux effects). Concurrent increases in pyruvate content would be consistent with further blockage of citrate cycle/ respiration, but did not appear to be accompanied by alanine/lactate fermentation or high ethanolic fermentation. Some degree of malate formation cannot be ruled out in this instance (as an NAD-generating end-product of hypoxic ‘glycolysis’), as short distance transfer and subsequent metabolism could occur without altering overall pool sizes (Figure 7). More likely, however, is the capacity for endosperm cells to minimize oxygen demand by decreasing metabolic activity and ATP use during starch storage in a ª Blackwell Publishing Ltd, The Plant Journal, (2005), 42, 69–83 Positional cues for resource partitioning 79 manner analog to legume seeds (Rolletschek et al., 2003), potato tubers (Geigenberger, 2003), and wheat grains (VanDongen et al., 2004). The lack of metabolic shifts toward fermentation indicates a well-coordinated dynamic adjustment of kernel metabolism to low O2 availability. Several lines of evidence indicate that starch biosynthesis is not oxygen-limited in the normally hypoxic maize endosperm. Increasing the O2 supply did not change either (i) the steady-state levels of ADP-glucose (Figure 7), (ii) the typically rate-limiting activity of ADP-glucose pyrophosphorylase, (iii) the 13C-labeling of ADP-glucose (Figure 8), or (iv) the flux of 14C-labeled sucrose into starch (Figure 8). Furthermore, decreases in oxygen availability to 1/10 of ambient levels only marginally reduced incorporation of 14 C-sucrose into starch (Figure 8c). Starch biosynthesis may well represent a key adaptation to the oxygen depletion in endosperms of developing grains. Points of support include, first, that the energy demand for starch biosynthesis is considerably less than that of protein or lipid (Vertregt and deVries, 1987). Second, starch biosynthesis could contribute to a potentially critical cycling of PPi and its associated energy. Under low-oxygen conditions, PPi can maintain sucrose metabolism via sucrose synthase þ UDP-glucose pyrophosphophosphorylase (Huber and Akazawa, 1986; Kavakli et al., 2000). Needed PPi could be generated at sites of immediate use in cytoplasm during starch biosynthesis, because of the presence of a uniquely adapted cytosolic form of ADP-glucose pyrophosphorylase in endosperm of maize and other cereals (Denyer et al., 1996; Sakulsingharoj et al., 2004; Salamone et al., 2002). Möhlmann et al. (1997) further suggested that ATP regulation of starch synthesis could shift precursor synthesis to the cytosol under conditions such as hypoxia, thus favoring recycling of PPi. A related adaptation to the low-oxygen environment of the endosperm environment may be that of sucrose synthase induction by hypoxia (Sachs et al., 1996; Subbaiah and Sachs, 2001; Zeng et al., 1998). Both SUS1 and SH1 isoforms are transcriptionally induced by low oxygen in maize [the SH1 ‘kernel form’ being most responsive to hypoxia (Zeng et al., 1998)], and O2 levels in the present study altered enzyme activity and levels of the UDG-glucose product (Figure 7). Sucrose cleavage by this reversible enzyme is associated with starch biosynthesis, ATP conservation, and enhanced sucrose/hexose ratios. Hypoxic induction of sucrose synthase could thus aid expected metabolite channeling toward starch, shift products of sucrose cleavage away from hexoses (each requiring an ATP to enter glycolysis), and further favor rising sucrose/hexose ratios (as result of a high km for sucrose). Still further increases in sucrose/hexose ratios may result from sucrose resynthesis in endosperm (Cheng, 1997). Together, these changes could provide substrates best adapted for respiration, biosynthesis, and compatible sugar signals in an oxygen-depleted structure (Koch, 1996, 2004; Koch et al., 2000; Zeng et al., ª Blackwell Publishing Ltd, The Plant Journal, (2005), 42, 69–83 1998). In contrast, oxygen availability clearly limits lipid biosynthesis. Our tracer experiments showed that elevated oxygen levels increased conversion of 13C-sucrose to acetylcoenzyme A precursors for lipid biosynthesis (Figure 8a), and markedly enhanced incorporation of 14C-sucrose into lipids (Figure 8e). This is consistent with the high energy demand for lipid biosynthesis itself. Similar data were shown for seeds of oilseed rape (Vigeolas et al., 2003) where lipid and starch storage is co-localized in the green embryos. Collectively, this suggests a general role of oxygen on the lipid/starch balance in seeds. Our data also point to a significant role for O2 and ATP in assimilate transfer to the primary sites of lipid deposition in embryos (discussed further below). These results, together with the greater ATP cost for synthesizing lipid and protein versus starch (Vertregt and deVries, 1987), indicate a pronounced adaptive advantage for storage of non-starch reserves in the most well-oxygenated regions at the kernel periphery (embryo, aleurone, and vitreous layers of endosperm). Assimilate partitioning to the maize embryo is affected by local oxygen/energy levels In general, assimilate partitioning is controlled by gene expression during the developmental process (Becraft, 2001; Motto et al., 2003). For example, genes coding for enzymes of glycolysis and fatty acid synthesis in maize embryos are coordinately upregulated during development (Lee et al., 2002). Such developmental control is known to be modulated by the metabolic status of tissues (‘coarse and fine control’; Koch, 1996; Wobus and Weber, 1999; Geigenberger, 2003; Scheible et al., 2004). Significant insights into mechanisms regulating assimilate partitioning toward grain embryos have been difficult to obtain. Here we demonstrate a causal link between local O2 availability, ATP levels (supply), and resource allocation within developing maize kernels. Elevated O2 supply allowing higher respiratory activity enhanced uptake of 14C-label into the embryo (Figure 8d) and its subsequent conversion to storage products (Figure 8e). Energy levels of kernels also increased (Figure 6), and bioluminescence imaging showed that ATP levels rose in the embryo-attached region of endosperm and in the endosperm face of the scutellum (Figure 6c,d), the latter is a primary route for assimilate transport into the embryo (Griffith et al., 1987; Matthys-Rochon et al., 1998; see Figure 9). These data indicate that embryo nutrition may depend on local ATP levels, particularly at the endosperm/ embryo interface. Although hexose uptake in this context appears passive, sucrose uptake is energy-dependent (Griffith et al., 1987) and can progress via sucrose transporters in the maize embryo (Lee et al., 2002). In addition, even passive entry of hexoses is coupled to energy 80 Hardy Rolletschek et al. requirements for their initial metabolism by hexokinases. Activity of these enzymes is typically low in maize kernels, and associated with lipid accumulation (Doehlert, 1990). Their activity is critical under oxygen limitation (Bouny and Saglio, 1996). Another way in which O2 could stimulate delivery of assimilates to the embryo is by raising ATP levels that may otherwise limit storage of proteins and/or lipids in this structure. In addition to these embryo-localized mechanisms of oxygen-responsive partitioning, elevated ATP levels within the embryo-surrounding region might play a role. Sugar composition is central to embryo nutrition (Griffith et al., 1987; Matthys-Rochon et al., 1998) and responsive to enzyme activities in the embryo-surrounding region. Invertases can be active in these sites (Bate et al., 2004), and are markedly sensitive to O2 (Zeng et al., 1999). Oxygen availability could thus affect delivery of the hexoses preferentially taken up by the embryo. The link between O2 supply, local ATP levels and 14C-uptake let us hypothesize that the energy state at the endosperm/embryo interface may exert a control function for partitioning of photoassimilates toward the embryo and, thus, for embryo growth. Two important insights arise from the novel combination of approaches used in this study. (These tools allowed a literally ‘new view’ of gradients across developing endosperm, their interrelationships, and the extent of their oxygen-limitation in vivo.) First, we show that oxygen depletion in cells of the interior of growing maize kernels is far more pervasive than previously recognized. Second, manipulation of the oxygen supply in the kernel interior markedly altered (i) energy status, (ii) ATP gradients, (iii) metabolite levels, (iv) assimilate partitioning to embryos, and (v) deposition of non-starch storage products. The significance of these findings is twofold. Although possibly coincidental, a distinct functional and evolutionary advantage can be envisioned for sites of processes most limited by internal oxygen depletion (lipid and protein deposition) to be located in peripheral tissues of the kernel (e.g. the embryo and vitreous outer layers of the maize endosperm). Second, data here and elsewhere suggest that the starch-type endosperm of maize and other grains is particularly well-adapted for deposition of storage reserves under localized oxygen depletion. Experimental procedures Plant growth Maize plants (Zea mays L.) were cultivated in a greenhouse in spring and summer under natural light supplemented with lamps to provide a 16/8-h photoperiod and an approximate light intensity of 800 lmol photons m)2 sec)1. Temperature was controlled between 28 and 32C. Kernels were harvested at distinct developmental stages (DAP) during the mid-light phase, snap-frozen in liquid N2 and stored at )80C until analyzed. Measurement of internal O2 concentration The O2 concentration inside kernels was measured using oxygensensitive microsensors in a procedure modified from that developed earlier for legume cotyledons by Rolletschek et al. (2002a). For maize, the intact ear was carefully moved into a fixed position and anchored firmly for subsequent micromanipulation. To allow entry of the microsensor to the seed, a small cut was made into the husks. Oxygen concentration below the husks was near atmospheric levels independent of light supply as was tested before. The microsensor was inserted into the intact kernel (remaining on the ear) using a micromanipulator. The site of sensor entry was sealed with silicone to prevent oxygen movement along microchannels. Measurements were carried out within 5 min. The O2 concentration is expressed in % of atmospheric saturation (21 kPa ¼ 100%). After measurement, the seed was dissected at the measured transect to identify the exact position of the sensor. Imaging of local ATP concentration Bioluminescence imaging was used to measure ATP distribution in cryosections of kernels. This technique allows the quantitative, histographical mapping of ATP, and was described in detail earlier (Borisjuk et al., 2003). Briefly, cryosections were prepared from snap-frozen kernels, and immersed into an enzyme solution linking ATP to the reaction of firefly luciferase. The enzyme emits photons with an intensity proportional to the content of ATP. The light emission was registered with a photon-counting system (Hamamatsu, Herrsching, Germany) linked to a microscope. ATP concentrations determined by bioluminescence are directly proportional to those determined by conventional HPLC [correlation coefficient 0.85; P < 0.01 (Borisjuk et al., 2003)]. Metabolite analysis Frozen plant material was rapidly weighed, immediately homogenized with an ice-cold mortar and pestle in liquid N2, and extracted with trichloroacetic acid as in Rolletschek et al. (2002a). Starch was determined in the remaining pellet as in Borisjuk et al. (2002). Aliquots of snap-frozen extracts were used for subsequent metabolite analyses. Free amino acids were measured by HPLC (Rolletschek et al., 2002b). Dissolved sugars were measured photometrically (Borisjuk et al., 2002). Adenine nucleotides were measured after derivatization by HPLC following the procedure of Haink and Deussen (2003) modified as follows: 20 ll of extract were incubated with 100 ll chloroacetaldehyde, and 880 ll buffer (62 mM citrate, 76 mM KH2PO4, pH 5.5) at 80C for 40 min. Derivatized samples were immediately cooled to 4C until analysis. Chromatographic separation within 4 min and fluorescence detection was carried out as described by Haink and Deussen (2003). Metabolites of glycolysis and the citrate cycle were measured by liquid chromatography coupled to mass spectrometry (LC-MS). Chromatography was conducted using a DX-500 ion chromatography system (Dionex, Sunnyvale, CA, USA). Separation was carried out on a Dionex AS11-HC column (2 · 250 mm) and an additional guard column (AG11-HC) at 30C. A binary gradient at a constant flow rate of 0.5 ml min)1 was applied using 100 mM sodium hydroxide (eluent B) and distilled water (eluent A). The gradient was produced by linear concentration changes: these were initiated with 20% B, raised to 34% B during the first 7 min, 70% B in the next 7 min, and to 100% B in the final 1 min. After holding at 100% B for 4 min, levels were returned to 20% B over a 2-min period and equilibrated for 10 min. Column effluents were directed to an ASRS-ULTRA (2-mm) ª Blackwell Publishing Ltd, The Plant Journal, (2005), 42, 69–83 Positional cues for resource partitioning 81 anion self-regenerating suppressor (Dionex) working in the external water mode (2 ml min)1) at 100 mA. After passing the conductivity cell, the effluent was split (1:1), and directed into the electrospray chamber. MS analysis was performed using a triple quadrupole (LC1200; Varian, Palo Alto, CA, USA) using the following parameters: ESI N2 pressure 51 psi, needle )5000 V, shield )200 V, drying gas 225C and 18 psi, detector voltage 1500 V, mass peak with 0.7 amu, scan time 2.5, and detection in the negative ion mode. Metabolites were measured in parallel using single-ion monitoring mode scanning the deprotonated ions [M–H]) except for the following (scanned masses in parenthesis): acetyl-coenzyme A (264.9), ADP-glucose (293.6), UDP-glucose (282), citrate/isocitrate (173), malate (115), phosphoenolpyruvate (166.7), and fructose-1,6-diphosphate (168.8). The validity of the method was checked using known standards (Sigma, St Louis, MO, USA) and by previous recovery experiments with different tissues/species (Rolletschek et al., 2004a,b). Relative quantification was carried out using scanned ion traces. Data were normalized to plant mg fresh weight and to an internal reference (13C-labeled pyruvate; Isotec, Miamisburg, OH, USA) added during the extraction of each sample. Determination of enzyme activities ADP-glucose pyrophosphorylase (AGPase; E.C. 2.7.7.27) and sucrose synthase (E.C. 2.4.1.13) activities were assayed using coupled spectrophotometric assays as described earlier (Rolletschek et al., 2002b). Enzyme assays were tested for substrate dependence and substrate and linearity with time and amount of extract. Histological methods Iodine staining was used to visualize starch granules (Borisjuk et al., 2002). Lipid staining was conducted using fresh, free-hand sections with Sudan B as described by Brundrett et al. (1991). Briefly, Sudan red B (Sigma) was dissolved in polyethylene glycol (400D; Sigma) by heating at 90C for 1 h, and an equal volume of 90% (v/v) glycerol was added. Sections were stained for 24 h at room temperature, then rinsed with deionized water, fixed in 70% ethanol, and photographed. Acknowledgements Oxygen treatment To study the effect of altered oxygen supply, kernels on an intact ear (enclosed within a transparent plastic chamber) were aerated with gas mixtures (combined by a multigas controller, Type 647B; MKS, Muenchen, Germany) containing atmospheric oxygen levels (about 21 vol.%, referred to as to 100%), 42 vol.% O2 (¼200%) and 2.1 vol.% O2 (¼10%, balanced by N2), respectively, at a flow rate of 2.5 l min)1. After a 6-h incubation, kernels were quickly removed, immediately frozen in liquid N2, and stored at )80C until used for analysis. The multigas controller was also used for stepwise increases in the external oxygen supply (see Figure 5). We are grateful to U. Tiemann, K. Lipfert and K. Blaschek for excellent technical assistance, C. Klukas and F. Schreiber for mapping metabolic networks as well as M. Hajirezaei for help with 14 C-isotope studies. Supplementary Material The following material is available from http://www. blackwellpublishing.com/products/journals/suppmat/TPJ/TPJ2352/ TPJ2352sm.htm Table S1. Primary metabolites and their response to changing O2 supply. Data are given as mean standard deviation. Labeling experiments For monitoring of in vivo fluxes, developing kernels on an intact ear were labeled with either stable (1-13CFru-sucrose; Omicron Biochemicals, South Benol, IN, USA) or radioactive (U-14C-sucrose; Amersham-Buchler, Freiburg, Germany) tracers, and treated with different gas mixtures as described above. Using a microsyringe, 3 ll of labeled 14C-sucrose (7.4 MBq ml)1) were injected into the endospermal conducting tissue of developing kernels (25 DAP) on intact ears. The site of needle entry was sealed with silicone to prevent gas exchange. After a 6-h incubation time during the light period, the kernels were removed and immediately frozen in liquid N2. Subsequently, an extraction procedure was carried out yielding a water/ ethanol-soluble fraction, starch fraction and the remaining pellet as described earlier (Rolletschek et al., 2002b). A modified method was used for the determination of label uptake by the embryo and partitioning into lipids. The kernels (30 DAP) were labeled and treated as above. After a 6-h incubation, the embryo was rapidly removed from the kernel, rinsed quickly in distilled water, and immediately frozen in liquid N2. After weighing, the kernels were extracted as in Bligh and Dyer (1959) giving a chloroform-soluble fraction (lipids), water/ methanol-soluble fraction and the remaining pellet. For determination of the labeling pattern within metabolic intermediates, 3 ll of labeled 13C-sucrose (200 mM) were injected into the endospermal conducting tissue of intact kernels (25 DAP) as above. After a 2-h incubation, the kernels were removed quickly, immediately frozen in liquid N2, and subsequently extracted using trichloroacetic acid as described above. Amounts of 13C-labeled acetyl-coenzyme A and ADP-glucose were quantified by mass spectrometry. ª Blackwell Publishing Ltd, The Plant Journal, (2005), 42, 69–83 References Aoki, N., Hirose, T., Takahashi, S., Ono, K., Ishimaru, K. and Ohsugi, R. (1999) Molecular cloning and expression analysis of a gene for a sucrose transporter in maize (Zea mays L.). Plant Cell Physiol. 40, 1072–1078. Baluka, F. and Kubica, C. (1992) Relationship between the content of the basic nuclear proteins, chromatin structure, rDNA transcription and cell size in different tissues of the maize root apex. J. Exp. Bot. 252, 991–996. Bate, N.J., Niu, X., Wang, Y., Reimann, K.S. and Helentjaris, T.G. (2004) An invertase inhibitor from maize localizes to the embryo surrounding region during early kernel development. Plant Physiol. 134, 246–254. Becraft, P.W. (2001) Cell fate specification in the cereal endosperm. Cell Dev. Biol. 12, 387–394. Bligh, E.G. and Dyer, W.J. (1959) A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37, 911– 917. Borisjuk, L., Walenta, S., Rolletschek, H., Mueller-Klieser, W., Wobus, U. and Weber, H. (2002) Spatial analysis of plant metabolism, sucrose imaging within Vicia faba cotyledons reveals specific developmental patterns. Plant J. 29, 521–530. Borisjuk, L., Rolletschek, H., Walenta, S., Panitz, R., Wobus, U. and Weber, H. (2003) Energy status and its control on embryogenesis of legumes: ATP distribution within Vicia faba embryos is developmentally regulated and correlated with photosynthetic capacity. Plant J. 36, 318–329. 82 Hardy Rolletschek et al. Bouny, J.M. and Saglio, P.H. (1996) Glycolytic flux and hexokinase activities in anoxic maize root tips acclimated by hypoxic pretreatment. Plant Physiol. 111, 187–194. Boyer, C.D. and Hannah, L.C. (2001) Kernel mutants of corn. In Specialty Corns, 2nd edn (Hallauer, A.R., ed.). Boca Raton, FL: CRC Press, pp. 1–32. Brangeon, J., Reyss, A. and Prioul, J.L. (1997) In situ detection of ADP glucose pyrophosphorylase expression during maize endosperm development. Plant Physiol. Biochem. 35, 847–858. Brundrett, M.C., Kendrick, B. and Peterson, C.A. (1991) Efficient lipid staining in plant material with Sudan red B or fluorol yellow o88 in polyethylene glycol-glycerol. Biotech. Histochem. 66, 111–116. Cheng, W.-H. (1997) Gene-expression analysis of acid invertase and sucrose-phosphate synthase in maize. PhD Dissertation, University of Florida, Florida, pp. 109–134. Choi, S.-B., Zhang, Y., Ito, H., Stephens, K., Winder, T., Edwards, G.E. and Okita, T.W. (1997) Increasing rice productivity by manipulation of starch biosynthesis during seed development. In Feeding a world population of more than eight billion people: a challenge to science (Waterford, J., Armstrong, D., Fowden, L. and Riley, R., eds). New York: Oxford University Press, pp. 137– 149. Chrispeels and Sadava (eds) (2003) Plants, Genes, and Crop Biotechnology. Boston, Toronto, London, Singapore: Jones and Vartlett Publishers. Denyer, K., Dunlap, F., Thorbjornsen, T., Keeling, P. and Smith, A.M. (1996) The major form of ADP-glucose pyrophosphorylase in maize endosperm is extraplastidial. Plant Physiol. 112, 779–785. Doehlert, D.C. (1990) Distribution of enzyme activities within the developing maize (Zea mays) kernel in relation to starch, oil and protein accumulation. Physiol. Plant. 78, 560–567. Dwyer, T., Rolletschek, H. and Schreiber, F. (2004) Representing experimental biological data in metabolic networks. Proceedings of the 2nd Asia-Pacific Bioinformatics Conference, Dunedin, New Zealand. Conferences in Research and Practice in Information, Vol. 29. Focks, N. and Benning, C. (1998) Wrinkled 1: a novel, low-seed-oil mutant of Arabidopsis with a deficiency in the seed-specific regulation of carbohydrate metabolism. Plant Physiol. 118, 91–101. Geigenberger, P. (2003) Response of plant metabolism to too little oxygen. Curr. Opin. Plant Biol. 6, 247–256. Giroux, M.J., Boyer, C., Feix, G. and Hannah, L.C. (1994) Coordinated transcriptional regulation of storage product genes in the maize endosperm. Plant Physiol. 106, 713–722. Greenway, H. and Gibbs, J. (2003) Mechanisms of anoxia tolerance in plant. II. Energy requirements for maintenance and energy distribution to essential processes. Funct. Plant Biol. 30, 999– 1036. Griffith, S.M., Jones, R.J. and Brenner, M.L. (1987) In vitro sugar transport in Zea mays L. Kernels II. Characteristics of sugar absorption and metabolism by isolated developing embryos. Plant Physiol. 84, 472–475. Haink, G. and Deussen, A. (2003) Liquid chromatography method for the analysis of adenosine compounds. J. Chromatogr. B, 784, 189–193. Hannah, L.C. and Greene, T.W. (1998) Maize seed weight is dependent on the amount of endosperm ADP-glucose pyrophosphorylase. J. Plant Physiol. 152, 649–652. Huber, S.C. and Akazawa, T. (1986) A novel sucrose synthase pathway for sucrose degradation in cultured sycamore cells. Plant Physiol. 81, 1008–1013. Hueros, G., Royo, J., Maitz, M., Salamini, F. and Thompson, R.D. (1999) Evidence for factors regulating transfer cell-specific expression in maize endosperm. Plant Mol. Biol. 41, 403–414. Jose-Estanyol, M., Ruiz-Avila, L. and Puigdomenech, P. (1992) A maize embryo-specific gene encodes a proline-rich hydrophobic protein. Plant Cell, 4, 413–423. Kavakli, I.H., Slattery, C.J., Ito, H. and Okita, T.W. (2000) The conversion of carbon and nitrogen into starch and storage proteins in developing storage organs: an overview. Aust. J. Plant Physiol. 27, 561–570. Killian, A., Heller, K. and Kleinhofs, A. (1998) Development patterns of telomerase activity in barley and maize. Plant Mol. Biol. 37, 621–628. Koch, K.E. (1996) Carbohydrate-modulated gene expression in plants. Ann. Rev. Plant Physiol. Plant Mol. Biol. 47, 509–540. Koch, K.E. (2004) Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr. Opin. Plant Biol. 7, 235–246. Koch, K.E., Ying, Z., Wu, Y. and Avigne, W.T. (2000) Multiple paths of sugar-sensing and a sugar/oxygen overlap for genes of sucrose and ethanol metabolism. J. Exp. Bot. 51, 417–427. Kondorosi, E., Roudier, F. and Gendreau, E. (2000) Plant cell-size control: growing by ploidy? Curr. Opin. Plant Biol. 3, 488–492. Kowles, R.V. and Phillips, R.L. (1988) Endosperm development in maize. Int. Rev. Cytol. 112, 97–136. Larkins, B.A., Dilkes, B.P., Dante, R.A., Coelho, C.M., Woo, Y.-M. and Liu, Y. (2001) Investigating the hows and whys of DNA endoreduplication. J. Exp. Bot. 52, 183–192. Lee, J.-M., Williams, M.E., Tingey, S.V. and Rafaalski, J.A. (2002) DNA array profiling of gene expression changes during maize embryo development. Funct. Integr. Genomics, 2, 13–27. Lin, Y., Sun, L, Nguyen, L.V., Rachubinski, R.A. and Goodman, H.M. (1999) The pex16b homolog SSE1 and storage organelle formation in Arabidopsis seeds. Science, 284, 328–330. Matthys-Rochon, E., Piola, F., LeDeunff, E., Mol, R. and Dumas, C. (1998) In vitro development of maize embryos: a tool for embryogenesis analysis. J. Exp. Bot. 49, 839–845. Möhlmann, T., Tjaden, J., Henrichs, G., Quick, W.P., Häusler, R. and Neuhaus, H.E. (1997) ADP-glucose drives starch synthesis in isolated maize endosperm amyloplasts: characterization of starch synthesis and transport properties across the amyloplast envelope. Biochem. J. 324, 503–509. Motto, M., Hartings, H. and Rossi, V. (2003) Gene discovery to improve the maize grain cell factory. Part 1. Agro Food Industry Hi-Tech, 14, 60–64. Nagl, W., Pohl, J. and Radler, A. (1985) The DNA endoreduplication cycles. In The Cell Division Cycle in Plants (Bryant, J.A. and Francis, D., eds). New York: Cambridge University Press, pp. 217– 232. Perdomo, A. and Burris, J.S. (1998) Histochemical, physiological, and ultrastructural changes in the maize embryo during artificial drying. Crop Sci. 38, 1236–1244. Rolletschek, H., Borisjuk, L., Koschorreck, M., Wobus, U. and Weber, H. (2002a) Legume embryos develop in a hypoxic environment. J. Exp. Bot. 53, 1099–1107. Rolletschek, H., Hajirezaei, M., Wobus, U. and Weber, H. (2002b) Antisense-inhibition of ADP-glucose pyrophosphorylase in Vicia narbonensis seeds increases soluble sugars and lead to higher water and nitrogen uptake. Planta, 214, 954–964. Rolletschek, H., Weber, H. and Borisjuk, L. (2003) Energy status and its control on embryogenesis of legumes. Embryo photosynthesis contributes to oxygen supply and is coupled to biosynthetic fluxes. Plant Physiol. 132, 1196–1206. Rolletschek, H., Weschke, W., Weber, H., Wobus, U. and Borisjuk, L. (2004a) Energy state and its control on seed development: starch accumulation is associated with high ATP and steep oxygen gradients within barley grains. J. Exp. Bot. 55, 1351–1359. ª Blackwell Publishing Ltd, The Plant Journal, (2005), 42, 69–83 Positional cues for resource partitioning 83 Rolletschek, H., Borisjuk, L., Radchuk, R., Miranda, M., Heim, U., Wobus, U. and Weber, H. (2004b) Seed-specific expression of a bacterial phosphoenolpyruvate carboxylase in Vicia narbonensis increases protein content and improves carbon economy. Plant Biotechnol. J. 2, 211–219. Ruuska, S.A., Schwender, J. and Ohlrogge, J.B. (2004) The capacity of green oilseeds to utilize photosynthesis to drive biosynthetic processes. Plant Physiol. 136, 1–10. Sachs, M.M., Subbaiah, C.C. and Saab, I.N. (1996) Anaerobic gene expression and flooding tolerance in maize. J. Exp. Bot. 47, 1–15. Sakulsingharoj, C., Choi, S., Hwang, S., Edwards, G., Borj, J., Meyer, C., Preiss, J. and Okita, T. (2004) Engineering starch biosynthesis for increasing rice seed weight: the role of the cytoplasmic ADPglucose pyrophosphorylase. Plant Sci. 167, 1323–1333. Salamone, P.R., Kavakli, I.H., Slattery, C.J. and Okita, T.W. (2002) Directed molecular evolution of ADP-glucose pyrophosphorylase. Proc. Natl Acad. Sci. USA, 99, 1070–1075. Scheible, W.-R., Morcuende, R., Czechowski, T., Fritz, C., Osuna, D., Palacios-Rojas, N., Schindelasch, D., Thimm, O., Udvardi, M.K. and Stitt, M. (2004) Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol. 136, 2483–2499. Schweizer, L., Yerk-Davies, G.L., Phillips, R.L., Srienc, F. and Jones, R.J. (1995) Dynamics of maize endosperm development and DNA endoreduplication. Proc. Natl Acad. Sci. USA, 92, 7070–7074. Shannon, J.C. (1972) Movement of 14C-labeled assimilates into kernels of Zea mays L. I. Pattern and rate of sugar movement. Plant Physiol. 49, 198–202. Shelp, B.J., Walton, C.S., Snedden, W.A., Tuin, L.G., Oresnik, I.J. and Layzell, D.B. (1995) Gaba shunt in developing soybean seeds is associated with hypoxia. Physiol. Plant. 94, 219–228. Stiefel, V., Becerra, E.L., Roca, R., Bastida, M., Jahrmann, T., Graziano, E. and Puigdomenech, P. (1999) TM20, a gene for a new class of transmembrane proteins expressed in the meristematic tissues of maize. J. Biol. Chem. 274, 27734–27739. Subbaiah, C.C. and Sachs, M.M. (2001) Molecular and cellular adaptations of maize to flooding stress. Ann. Bot., 91, 119–127. Thomas, P.A., Felker, F.C. and Crawford, C.G. (1992) Sugar uptake and metabolism in the developing endosperm of Tasselseed-Tu (Ts5-Tu). Plant Physiol. 99, 1540–1546. Trethewey, R.N., Geigenberger, P., Hennig, A., Fleischer-Noter, H., Muller-Rober, B. and Willmitzer, L. (1999) Induction of the activity of glycolytic enzymes correlates with enhanced hydrolysis of sucrose in the cytosol of transgenic potato tubers. Plant Cell Environ. 22, 71–79. Vance, V.B. and Huang, A.H.C. (1988) Expression of lipid body protein gene during maize seed development. J. Biol. Chem. 263, 1476–1481. ª Blackwell Publishing Ltd, The Plant Journal, (2005), 42, 69–83 VanDongen, J.T., Schurr, U., Pfister, M. and Geigenberger, G. (2003) Phloem metabolism and function have to cope with low internal oxygen. Plant Physiol. 131, 1529–1543. VanDongen, J.T., Roeb, G.W., Dautzenberg, M., Froehlich, A., Vigeolas, H., Minchin, P.E.H. and Geigenberger, P. (2004) Phloem import and storage metabolism are highly coordinated by the low oxygen concentrations within developing wheat seeds. Plant Physiol. 135, 1809–1821. Vertregt, N. and deVries, P. (1987) A rapid method for determining the efficiency of biosynthesis of plant biomass. J. Theor. Biol. 128, 109–110. Vigeolas, H., van Dongen, J.T., Waldeck, P., Hühn, D. and Geigenberger, P. (2003) Lipid storage metabolism is limited by the prevailing low oxygen concentrations within developing seeds of oilseed rape. Plant Physiol. 133, 2048–2060. Vilhar, B., Kladnik, A., Blejec, A., Chourey, P.S. and Dermastia, M. (2002) Cytometrical evidence that the loss of seed weight in the miniature1 seed mutant of maize is associated with reduced mitotic activity in the developing endosperm. Plant Physiol. 129, 23–30. Wager, H.G. (1974) The effect of subjecting peas to air enriched with carbon dioxide I. The path of gaseous diffusion, the content of CO2 and the buffering of the tissue. J. Exp. Bot. 25, 330–337. Winter, H. and Huber, S.C. (2000) Regulation of sucrose metabolism in higher plants: Localization and regulation of activity of key enzymes. Critical Reviews in Biochemistry and Molecular Biology 35(4), 253–289. Wobus, U. and Weber, H. (1999) Sugars as signal molecules in plant seed development. Biol. Chem. 380, 937–944. Wobus, U., Sreenivasulu, N., Borisjuk, L., Rolletschek, H., Panitz, R., Gubatz, S. and Weschke, W. (2004) Molecular physiology and genomics of developing barley grains. Recent Res. Dev. Plant Mol. Biol. 2, 1–29. Xia, J.-H. and Saglio, P. (1990) Hþ efflux and hexose transport under imposed energy statusinmaizeroottips.Plant Physiol. 93,453–459. Young, T.E., Gallie, D.R. and DeMason, D.A. (1997) Ethylenemediated programmed cell death during maize endosperm development of wild-type and shrunken2 genotypes. Plant Physiol. 115, 737–751. Zeng, Y., Wu, Y., Avigne, W.T. and Koch, K.E. (1998) Differential regulation of sugar-sensitive sucrose synthases by hypoxia and anoxia indicate complementary transcriptional and posttranscriptional responses. Plant Physiol. 116, 1573–1583. Zeng, Y., Wu, Y., Avigne, W.T. and Koch, K.E. (1999) Rapid repression of maize invertases by low oxygen. Invertase/sucrose synthase balance, sugar signaling potential, and seedling survival. Plant Physiol. 121, 599–608.