Biochemistry Notes
... 1. Tetravalent - can form four bonds with other elements 2. May form double bonds and triple bonds. 3. Bond with itself forming Chains of various lengths 4. The chains may branch. 5. May form rings ...
... 1. Tetravalent - can form four bonds with other elements 2. May form double bonds and triple bonds. 3. Bond with itself forming Chains of various lengths 4. The chains may branch. 5. May form rings ...
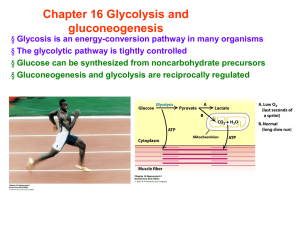
Chapter 16 Glycolysis and gluconeogenesis
... Chapter 16 Glycolysis and gluconeogenesis § Glycosis is an energy-conversion pathway in many organisms § The glycolytic pathway is tightly controlled § Glucose can be synthesized from noncarbohydrate precursors § Gluconeogenesis and glycolysis are reciprocally regulated ...
... Chapter 16 Glycolysis and gluconeogenesis § Glycosis is an energy-conversion pathway in many organisms § The glycolytic pathway is tightly controlled § Glucose can be synthesized from noncarbohydrate precursors § Gluconeogenesis and glycolysis are reciprocally regulated ...
Nutrition Power Point
... You will notice that even after you have finished racing you will continue to breath hard. At this point your body is still trying to repay the oxygen debt that was created when you were working hard. Technically, it is excessive post-exercise oxygen consumption (EPOC). ...
... You will notice that even after you have finished racing you will continue to breath hard. At this point your body is still trying to repay the oxygen debt that was created when you were working hard. Technically, it is excessive post-exercise oxygen consumption (EPOC). ...
Chapter 2: Atoms, Molecules, and Ions
... 12. The chemist credited for inventing a set of symbols for writing elements and a system for writing the formulas of compounds (and for discovering selenium, silicon, and thorium) is A) Boyle B) Lavoisier C) Priestly D) Berzelius E) Dalton 13. Avogadro's hypothesis states that: A) Each atom of oxyg ...
... 12. The chemist credited for inventing a set of symbols for writing elements and a system for writing the formulas of compounds (and for discovering selenium, silicon, and thorium) is A) Boyle B) Lavoisier C) Priestly D) Berzelius E) Dalton 13. Avogadro's hypothesis states that: A) Each atom of oxyg ...
Chapter 14 Glycolysis and the catabolism of hexoses
... 2 pyruvate + 2 NADH + 2H+ + 2ADP + 4 ATP + 2H2O for a net of Glucose + 2NAD+ + 2ADP + 2Pi 6 2 pyruvate + 2 NADH + 2H+ + 2ATP + 2H2O Under aerobic conditions the 2 NADH are transferred to the mitochondria where the can be changed back to NAD+ and, in the process generates additional ATP via respirati ...
... 2 pyruvate + 2 NADH + 2H+ + 2ADP + 4 ATP + 2H2O for a net of Glucose + 2NAD+ + 2ADP + 2Pi 6 2 pyruvate + 2 NADH + 2H+ + 2ATP + 2H2O Under aerobic conditions the 2 NADH are transferred to the mitochondria where the can be changed back to NAD+ and, in the process generates additional ATP via respirati ...
1 - Medical Mastermind Community
... Electrons flow from least reduced to most reduced carriers. The rate of electron transport is controlled by the concentration of ADP. The transfer of electrons from FADH2 to NAD+ is thermodynamically favored. The transfer of electrons from the cytosol to the mitochondrion by the glycerol phosphate s ...
... Electrons flow from least reduced to most reduced carriers. The rate of electron transport is controlled by the concentration of ADP. The transfer of electrons from FADH2 to NAD+ is thermodynamically favored. The transfer of electrons from the cytosol to the mitochondrion by the glycerol phosphate s ...
Biomolecules I. Introduction. - biochemistry: study of chemical
... - enzymes are globular proteins, act as biological catalysts; they cannot force a reaction to occur, only accelerate rate at which it proceeds. - some enzymes are just globular proteins, others consist of proteins and cofactors. - enzymes are highly specific, usually involved in control of one chemi ...
... - enzymes are globular proteins, act as biological catalysts; they cannot force a reaction to occur, only accelerate rate at which it proceeds. - some enzymes are just globular proteins, others consist of proteins and cofactors. - enzymes are highly specific, usually involved in control of one chemi ...
File - Manthey AP Biology
... ATP (adenosine triphosphate) is the cell’s renewable and reusable energy shuttle ATP provides energy for cellular functions Energy to charge ATP comes from catabolic reactions ...
... ATP (adenosine triphosphate) is the cell’s renewable and reusable energy shuttle ATP provides energy for cellular functions Energy to charge ATP comes from catabolic reactions ...
Chapter 8 Metabolism APc8metabolismme (1)
... ATP (adenosine triphosphate) is the cell’s renewable and reusable energy shuttle ATP provides energy for cellular functions Energy to charge ATP comes from catabolic reactions ...
... ATP (adenosine triphosphate) is the cell’s renewable and reusable energy shuttle ATP provides energy for cellular functions Energy to charge ATP comes from catabolic reactions ...
An endosperm enzyme catalyzes the formation of phosphotriester
... nucleotides including polyU, polyC and polyA had been tested as substrates for demonstrating the catalysis of enzyme reaction. However, the minimum requirement of at least 3 to 4 unites of nucleotides chain length in order to be able initiating the enzyme action suggests that the only phosphate grou ...
... nucleotides including polyU, polyC and polyA had been tested as substrates for demonstrating the catalysis of enzyme reaction. However, the minimum requirement of at least 3 to 4 unites of nucleotides chain length in order to be able initiating the enzyme action suggests that the only phosphate grou ...
ENERGY CURRENCY
... order for it to be utilized, it first must be converted into ATP. In order for this conversion to occur, oxidative pathways must be available. NAD+ is nicotinamide adenine dinucleotide and is found in all cells. It is actually classified as a coenzyme . In its reduced high energy form it is official ...
... order for it to be utilized, it first must be converted into ATP. In order for this conversion to occur, oxidative pathways must be available. NAD+ is nicotinamide adenine dinucleotide and is found in all cells. It is actually classified as a coenzyme . In its reduced high energy form it is official ...
1. This cartoon shows Complex I in the ETC, in its two alternative
... d. In the “empty egg” below, use dots or shading to predict the distribution of bicoid protein. 2pts NOTE: Concentration (dots) should be focused at anterior edge and lessen on a gradient to the posterior edge. Answers were largely correct or incorrect, but partial credit was possible if the dots we ...
... d. In the “empty egg” below, use dots or shading to predict the distribution of bicoid protein. 2pts NOTE: Concentration (dots) should be focused at anterior edge and lessen on a gradient to the posterior edge. Answers were largely correct or incorrect, but partial credit was possible if the dots we ...
Ch. 6 PPT
... 6.10 Most ATP production occurs by oxidative phosphorylation • Electrons from NADH and FADH2 – Travel down the electron transport chain to oxygen, which picks up H+ to form water • Energy released by the redox reactions ...
... 6.10 Most ATP production occurs by oxidative phosphorylation • Electrons from NADH and FADH2 – Travel down the electron transport chain to oxygen, which picks up H+ to form water • Energy released by the redox reactions ...
Most common elements in living things are carbon, hydrogen
... Carbohydrates are used by the body for energy and structural support in cell walls of plants and exoskeletons of insects and crustaceans. They are made of smaller subunits called monosaccharides. Monosaccharides have carbon, hydrogen, and oxygen in a 1:2:1 ratio. Monosaccharides or simple sugars inc ...
... Carbohydrates are used by the body for energy and structural support in cell walls of plants and exoskeletons of insects and crustaceans. They are made of smaller subunits called monosaccharides. Monosaccharides have carbon, hydrogen, and oxygen in a 1:2:1 ratio. Monosaccharides or simple sugars inc ...
basic biochemistry - Personal Webspace for QMUL
... These are at the two positions where ATP is formed 1: The 1,3-Bisphosphoglycerate to 3-Phosphoglycerate The 1,3-BPG passes a phosphate to ADP This is known as substrate-level phosphorylation ...
... These are at the two positions where ATP is formed 1: The 1,3-Bisphosphoglycerate to 3-Phosphoglycerate The 1,3-BPG passes a phosphate to ADP This is known as substrate-level phosphorylation ...
Glycolysis
... 2 NADH to 2 NAD+ (Lactic acid fermentation ) = 0 ATP 2 Pyruvate to 2 Lactate (Lactic acid fermentation) = 0 ATP Total yield in anaerobic respiration = 2 ATP ...
... 2 NADH to 2 NAD+ (Lactic acid fermentation ) = 0 ATP 2 Pyruvate to 2 Lactate (Lactic acid fermentation) = 0 ATP Total yield in anaerobic respiration = 2 ATP ...
24.t Glycolysis
... comes from inorganic phosphate ions present in the cytoplasm, so no ATP is expended here. In fact, I,3-bisphosphoglycerateis itself a high-energy compound-a mixed anhydride of a carboxylic acid and phosphoric acid (see Sec.23.7) that can transfer its newphosphoryl group to ADP (The phosphorylation o ...
... comes from inorganic phosphate ions present in the cytoplasm, so no ATP is expended here. In fact, I,3-bisphosphoglycerateis itself a high-energy compound-a mixed anhydride of a carboxylic acid and phosphoric acid (see Sec.23.7) that can transfer its newphosphoryl group to ADP (The phosphorylation o ...
Jeopardy
... how much carbon is used by plants During the daytime. What control can Be used in this experiment? ...
... how much carbon is used by plants During the daytime. What control can Be used in this experiment? ...
Chapter 6
... Very similar to aerobic respiration in eukaryotes Since prokaryotes have no mitochondria, it all occurs in the cytoplasm. Makes 2 more ATP because the NADH from glycolysis isn’t converted to FADH2 ...
... Very similar to aerobic respiration in eukaryotes Since prokaryotes have no mitochondria, it all occurs in the cytoplasm. Makes 2 more ATP because the NADH from glycolysis isn’t converted to FADH2 ...
Metal Ion Transport and Storage
... – High charge density cations require help • Once inside the cell, metal ions must be transported to the location of their use, then released or stored for later – Release from ligand is often not trivial – Storage requires additional molecules ...
... – High charge density cations require help • Once inside the cell, metal ions must be transported to the location of their use, then released or stored for later – Release from ligand is often not trivial – Storage requires additional molecules ...
THINK-PAIR
... • Where does CR take place in prokaryotes and in eukaryotes? • Explain the role of an electrochemical gradient in forming ATP molecules. • What would happen with the process of cellular respiration if mitochondria would be punctured? ...
... • Where does CR take place in prokaryotes and in eukaryotes? • Explain the role of an electrochemical gradient in forming ATP molecules. • What would happen with the process of cellular respiration if mitochondria would be punctured? ...
CO-ENZYMES i.
... catalysts that speed up the pace of chemical reactions. 2. A chemical reaction without an enzyme is like a drive over a mountain. The enzyme bores a tunnel through it so that passage is far quicker and takes much less energy. 3. Enzymes make life on earth possible, all biology from conception to the ...
... catalysts that speed up the pace of chemical reactions. 2. A chemical reaction without an enzyme is like a drive over a mountain. The enzyme bores a tunnel through it so that passage is far quicker and takes much less energy. 3. Enzymes make life on earth possible, all biology from conception to the ...
Energy Systems
... responsible for increases in acidity in the muscle. High acidity is one factor that contributes to acute muscular discomfort experienced during and shortly after intense exercise. However, recent evidence suggests fatigue is caused by calcium leaking into muscle cells from release channels within th ...
... responsible for increases in acidity in the muscle. High acidity is one factor that contributes to acute muscular discomfort experienced during and shortly after intense exercise. However, recent evidence suggests fatigue is caused by calcium leaking into muscle cells from release channels within th ...
Assay the Activity of Alkaline Phosphatase (ALP) in Serum
... Enzymes in clinical diagnosis • An enzyme test is a blood test or urine test that measures levels of certain enzymes to assess how well the body’s systems are functioning and whether there has been any tissue damage. (why?) ...
... Enzymes in clinical diagnosis • An enzyme test is a blood test or urine test that measures levels of certain enzymes to assess how well the body’s systems are functioning and whether there has been any tissue damage. (why?) ...
PowerPoint Presentation - Nerve activates contraction
... ◦ Your body breaks down the glycogen and releases glucose to your blood ...
... ◦ Your body breaks down the glycogen and releases glucose to your blood ...
Oxidative phosphorylation
Oxidative phosphorylation (or OXPHOS in short) is the metabolic pathway in which the mitochondria in cells use their structure, enzymes, and energy released by the oxidation of nutrients to reform ATP. Although the many forms of life on earth use a range of different nutrients, ATP is the molecule that supplies energy to metabolism. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is probably so pervasive because it is a highly efficient way of releasing energy, compared to alternative fermentation processes such as anaerobic glycolysis.During oxidative phosphorylation, electrons are transferred from electron donors to electron acceptors such as oxygen, in redox reactions. These redox reactions release energy, which is used to form ATP. In eukaryotes, these redox reactions are carried out by a series of protein complexes within the inner membrane of the cell's mitochondria, whereas, in prokaryotes, these proteins are located in the cells' intermembrane space. These linked sets of proteins are called electron transport chains. In eukaryotes, five main protein complexes are involved, whereas in prokaryotes many different enzymes are present, using a variety of electron donors and acceptors.The energy released by electrons flowing through this electron transport chain is used to transport protons across the inner mitochondrial membrane, in a process called electron transport. This generates potential energy in the form of a pH gradient and an electrical potential across this membrane. This store of energy is tapped by allowing protons to flow back across the membrane and down this gradient, through a large enzyme called ATP synthase; this process is known as chemiosmosis. This enzyme uses this energy to generate ATP from adenosine diphosphate (ADP), in a phosphorylation reaction. This reaction is driven by the proton flow, which forces the rotation of a part of the enzyme; the ATP synthase is a rotary mechanical motor.Although oxidative phosphorylation is a vital part of metabolism, it produces reactive oxygen species such as superoxide and hydrogen peroxide, which lead to propagation of free radicals, damaging cells and contributing to disease and, possibly, aging (senescence). The enzymes carrying out this metabolic pathway are also the target of many drugs and poisons that inhibit their activities.