
Cellular Respiration/Fermentation Review Sheet
... 10. What do plants do with the CO2 produced during cellular respiration? THEY STORE IT IN THEIR CELLS & USE IT FOR PHOTOSYNTHESIS 11. What do we do with the H2O produced when glucose breaks down? STORE IT, USE IT TO MAINTAIN CELL HEALTH, OR EXCRETE AS A WASTE PRODUCT 12. What do plants do with the H ...
... 10. What do plants do with the CO2 produced during cellular respiration? THEY STORE IT IN THEIR CELLS & USE IT FOR PHOTOSYNTHESIS 11. What do we do with the H2O produced when glucose breaks down? STORE IT, USE IT TO MAINTAIN CELL HEALTH, OR EXCRETE AS A WASTE PRODUCT 12. What do plants do with the H ...
PASSIVE TRANSPORT
... Diffusion, Osmosis and electrochemical gradient OSMOSIS: It is a phenomenon in which there is a flow of solvent (usually water) between two solutions separated by a semipermeable membrane; the phenomenon is generally due to concentration differences, and in that case the solvent flows from the less ...
... Diffusion, Osmosis and electrochemical gradient OSMOSIS: It is a phenomenon in which there is a flow of solvent (usually water) between two solutions separated by a semipermeable membrane; the phenomenon is generally due to concentration differences, and in that case the solvent flows from the less ...
Chemical digestion
... (finger like projections). Absorbed by blood. Absorption of fats occurs at the villi; absorbed into lymph system. ...
... (finger like projections). Absorbed by blood. Absorption of fats occurs at the villi; absorbed into lymph system. ...
Cellular Respiration
... • This is aerobic cellular respiration. • In aerobic respiration, glucose is broken down completely into ATP, carbon dioxide and water. ...
... • This is aerobic cellular respiration. • In aerobic respiration, glucose is broken down completely into ATP, carbon dioxide and water. ...
Bio 6B Lecture Slides - B
... One of the great mysteries of the origin of living cells — • All non-biological synthesis reactions of organic molecules produce both D- and L- isomers in equal yield. • And all non-biological reactions using organic molecules as reactants react with both D- and L- isomers equally. • Yet, living cel ...
... One of the great mysteries of the origin of living cells — • All non-biological synthesis reactions of organic molecules produce both D- and L- isomers in equal yield. • And all non-biological reactions using organic molecules as reactants react with both D- and L- isomers equally. • Yet, living cel ...
Anorexia Nervosa Anorexia Nervosa
... Purpose is to build a negative charge inside membrane and thus attract H+ ions back into the mitochondrion. (the membrane is impervious to H+ except for proton channels; therefore the protons have no choice but to operate the ATPase enzyme) Oxidative Phosphorylation: The conversion of ADP and inorga ...
... Purpose is to build a negative charge inside membrane and thus attract H+ ions back into the mitochondrion. (the membrane is impervious to H+ except for proton channels; therefore the protons have no choice but to operate the ATPase enzyme) Oxidative Phosphorylation: The conversion of ADP and inorga ...
Handout: Fatty Acid Synthesis
... Insulin stimulates FA synthesis by stimulating protein phosphatase that activates acetyl CoA carboxylase. ...
... Insulin stimulates FA synthesis by stimulating protein phosphatase that activates acetyl CoA carboxylase. ...
Chemistry 1st Semester Practice Exam
... 56. Which of the following compounds would you expect to be ionic? A. H2O B. CO2 51. Which group of elements is most likely to form ions by losing one electron? ...
... 56. Which of the following compounds would you expect to be ionic? A. H2O B. CO2 51. Which group of elements is most likely to form ions by losing one electron? ...
Finals Practice Exam
... phosphorylation, such that if oxidative phosphorylation is inhibited, the TCA cycle will also be inhibited? ...
... phosphorylation, such that if oxidative phosphorylation is inhibited, the TCA cycle will also be inhibited? ...
supporting information
... The net energy of butyrate (in the form of ATP, molTP/ (L·h)) is (3* VHBu – 1/4*(VBu + DiffBu * (H+/(H+ + 10-pKa,HBu)) * VHBu * X /D)), in which 3 is ATP yield per butyrate from glucose; 1/4 is ATP consumed for proton motive force. Therefore, the ATP net of butyrate yield is shorten as f_Bu * vP. An ...
... The net energy of butyrate (in the form of ATP, molTP/ (L·h)) is (3* VHBu – 1/4*(VBu + DiffBu * (H+/(H+ + 10-pKa,HBu)) * VHBu * X /D)), in which 3 is ATP yield per butyrate from glucose; 1/4 is ATP consumed for proton motive force. Therefore, the ATP net of butyrate yield is shorten as f_Bu * vP. An ...
Dark Reactions
... Recall the oxygenase activity of rubisco. Under normal conditions the rate of the carboxylase reaction is 4 times faster than the oxygenase reaction. Normal conditions being P = 1 atm, T = 25 oC, [CO2] = 10 µM and [O2] = 250 µM. When the temperature increases the rate of the oxygenase activity incre ...
... Recall the oxygenase activity of rubisco. Under normal conditions the rate of the carboxylase reaction is 4 times faster than the oxygenase reaction. Normal conditions being P = 1 atm, T = 25 oC, [CO2] = 10 µM and [O2] = 250 µM. When the temperature increases the rate of the oxygenase activity incre ...
medbiochem exam 1, 2000
... 47. Which of the following statements is TRUE? Enzyme catalysis of a chemical reaction: A. increases the energy of the transition state. B. decreases the change in free energy of the reaction. C. increases the change in free energy of the reaction. D. decreases the change in the free energy of activ ...
... 47. Which of the following statements is TRUE? Enzyme catalysis of a chemical reaction: A. increases the energy of the transition state. B. decreases the change in free energy of the reaction. C. increases the change in free energy of the reaction. D. decreases the change in the free energy of activ ...
FACTORS AFFECTING ENZYME ACTION
... • 3-D structure - the amino acid sequence causes bonds to form and the polypeptide to fold into a 3D shape. • The 3D shape causes the enzyme to form an active site - this is a “hole” that forms and is able to bind onto other molecules by forming temporary bonds between them. • The molecule that the ...
... • 3-D structure - the amino acid sequence causes bonds to form and the polypeptide to fold into a 3D shape. • The 3D shape causes the enzyme to form an active site - this is a “hole” that forms and is able to bind onto other molecules by forming temporary bonds between them. • The molecule that the ...
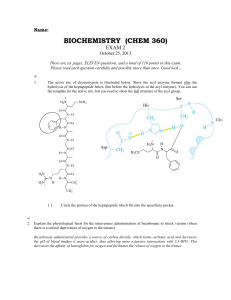
BIOCHEMISTRY (CHEM 360)
... Explain the physiological basis for the intravenous administration of bicarbonate to shock victims (when there is a critical deprivation of oxygen to the tissues) Bicarbonate administered provides a source of carbon dioxide, which forms carbonic acid and decreases the pH of blood (makes it more acid ...
... Explain the physiological basis for the intravenous administration of bicarbonate to shock victims (when there is a critical deprivation of oxygen to the tissues) Bicarbonate administered provides a source of carbon dioxide, which forms carbonic acid and decreases the pH of blood (makes it more acid ...
The Chemical Context of Life by Dr. Ty C.M. Hoffman
... measure of an atom's degree to which it attracts electrons is called its electronegativity. If a covalent bond is formed between two atoms that have drastically different electronegativities, the shared elect ...
... measure of an atom's degree to which it attracts electrons is called its electronegativity. If a covalent bond is formed between two atoms that have drastically different electronegativities, the shared elect ...
Fundamentals: Bioenergetics and Enzyme Function
... 5. Why is the D and I structure interpretation of glycogen synthetase activity no longer acceptable? 6. Understand and apply the terminology of the A0.5 for G6P and the S0.5 for UDPglucose to the in vivo regulation of synthetase? 7. Do the enzyme activation curves for synthetase indicate Michaelis M ...
... 5. Why is the D and I structure interpretation of glycogen synthetase activity no longer acceptable? 6. Understand and apply the terminology of the A0.5 for G6P and the S0.5 for UDPglucose to the in vivo regulation of synthetase? 7. Do the enzyme activation curves for synthetase indicate Michaelis M ...
U5Word
... B. FA synthase: in E Coli, this consists of a number of separate enzymes, but in animals 2 identical subunits each contain the enzyme activities for all the rxns ( oxidation has a different enzyme for each step). 1. The substrate remains bound to the long phosphopantethein prosthetic group (Fig. 25 ...
... B. FA synthase: in E Coli, this consists of a number of separate enzymes, but in animals 2 identical subunits each contain the enzyme activities for all the rxns ( oxidation has a different enzyme for each step). 1. The substrate remains bound to the long phosphopantethein prosthetic group (Fig. 25 ...
[j26]Chapter 5#
... respiration and to analyze the combustion reactions that occur continuously in all living cells. In this way, food consumption provides the high-energy raw materials that active cells require to produce the ATP that drives the cell’s activities and that ultimately maintains overall body homeostasis. ...
... respiration and to analyze the combustion reactions that occur continuously in all living cells. In this way, food consumption provides the high-energy raw materials that active cells require to produce the ATP that drives the cell’s activities and that ultimately maintains overall body homeostasis. ...
Biology: Cellular Respiration Practice Problems
... 14. On average, how many ATP can be made from each NADH during the electron transport process? 15. On average, how many ATP can be made from each FADH2 during the electron transport process? 16. What would happen to the cellular respiration process if the enzyme for one step of the process were miss ...
... 14. On average, how many ATP can be made from each NADH during the electron transport process? 15. On average, how many ATP can be made from each FADH2 during the electron transport process? 16. What would happen to the cellular respiration process if the enzyme for one step of the process were miss ...
What are Tetrahymena? - Department of Biological Sciences
... GTP Receptors • Cold GTP doesn’t compete with hot ATP for binding (and vice-versa) • No cross-adaptation (behavior and binding) • ATP responses are inhibited by pertussis toxin, calphostin C and Rp-cAMPS but not GTP responses • The ATP receptor may be metabotropic (P2Y-like?) and the GTP receptor io ...
... GTP Receptors • Cold GTP doesn’t compete with hot ATP for binding (and vice-versa) • No cross-adaptation (behavior and binding) • ATP responses are inhibited by pertussis toxin, calphostin C and Rp-cAMPS but not GTP responses • The ATP receptor may be metabotropic (P2Y-like?) and the GTP receptor io ...
Workbook
... _____ 2. C6H12O6 + 6O2 → 6CO2 + 6H2O is the chemical reaction of photosynthesis. _____ 3. Glucose is a carbohydrate that stores chemical energy in a concentrated and stable form. _____ 4. Only autotrophs can perform photosynthesis. _____ 5. Only four types of organisms — plants, algae, fungi and som ...
... _____ 2. C6H12O6 + 6O2 → 6CO2 + 6H2O is the chemical reaction of photosynthesis. _____ 3. Glucose is a carbohydrate that stores chemical energy in a concentrated and stable form. _____ 4. Only autotrophs can perform photosynthesis. _____ 5. Only four types of organisms — plants, algae, fungi and som ...
CHAPTER 6
... Figure 15.5 The isozymes of lactate dehydrogenase (LDH). Active muscle tissue becomes anaerobic and produces pyruvate from glucose via glycolysis (Chapter 18). It needs LDH to regenerate NAD+ from NADH so glycolysis can continue. The lactate produced is released into the blood. The muscle LDH isozy ...
... Figure 15.5 The isozymes of lactate dehydrogenase (LDH). Active muscle tissue becomes anaerobic and produces pyruvate from glucose via glycolysis (Chapter 18). It needs LDH to regenerate NAD+ from NADH so glycolysis can continue. The lactate produced is released into the blood. The muscle LDH isozy ...
Chapter 8
... 37oC reactants do not reach EA (transition state) Enzymes speed rate to transition state ...
... 37oC reactants do not reach EA (transition state) Enzymes speed rate to transition state ...
213lec3
... A. Metabolic pathways are sequences of reactions that are catalyzed by enzymes. Metabolic pathways allow for the slow and controlled breakdown of foodstuffs so that energy can be captured and used to form ATP. B. Glycolysis: Breaks glucose into two pyruvate molecules. After glycolysis but before ent ...
... A. Metabolic pathways are sequences of reactions that are catalyzed by enzymes. Metabolic pathways allow for the slow and controlled breakdown of foodstuffs so that energy can be captured and used to form ATP. B. Glycolysis: Breaks glucose into two pyruvate molecules. After glycolysis but before ent ...
Oxidative phosphorylation
Oxidative phosphorylation (or OXPHOS in short) is the metabolic pathway in which the mitochondria in cells use their structure, enzymes, and energy released by the oxidation of nutrients to reform ATP. Although the many forms of life on earth use a range of different nutrients, ATP is the molecule that supplies energy to metabolism. Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is probably so pervasive because it is a highly efficient way of releasing energy, compared to alternative fermentation processes such as anaerobic glycolysis.During oxidative phosphorylation, electrons are transferred from electron donors to electron acceptors such as oxygen, in redox reactions. These redox reactions release energy, which is used to form ATP. In eukaryotes, these redox reactions are carried out by a series of protein complexes within the inner membrane of the cell's mitochondria, whereas, in prokaryotes, these proteins are located in the cells' intermembrane space. These linked sets of proteins are called electron transport chains. In eukaryotes, five main protein complexes are involved, whereas in prokaryotes many different enzymes are present, using a variety of electron donors and acceptors.The energy released by electrons flowing through this electron transport chain is used to transport protons across the inner mitochondrial membrane, in a process called electron transport. This generates potential energy in the form of a pH gradient and an electrical potential across this membrane. This store of energy is tapped by allowing protons to flow back across the membrane and down this gradient, through a large enzyme called ATP synthase; this process is known as chemiosmosis. This enzyme uses this energy to generate ATP from adenosine diphosphate (ADP), in a phosphorylation reaction. This reaction is driven by the proton flow, which forces the rotation of a part of the enzyme; the ATP synthase is a rotary mechanical motor.Although oxidative phosphorylation is a vital part of metabolism, it produces reactive oxygen species such as superoxide and hydrogen peroxide, which lead to propagation of free radicals, damaging cells and contributing to disease and, possibly, aging (senescence). The enzymes carrying out this metabolic pathway are also the target of many drugs and poisons that inhibit their activities.
















![[j26]Chapter 5#](http://s1.studyres.com/store/data/013325615_1-93c4a55e793afe312f3604738fdd639e-300x300.png)





