Intracellular Respiration
... Hydrogen acceptors 1. NAD+ (oxidized) Nicotinamide adenine Dinucleotide, accepts electrons and becomes 2. NADH (reduced) ...
... Hydrogen acceptors 1. NAD+ (oxidized) Nicotinamide adenine Dinucleotide, accepts electrons and becomes 2. NADH (reduced) ...
Lab 11
... Introduction ( Why the experiment is important? ) – State a hypothesis (an “if then” statement, may require multiple sentences) that is clear and appropriately addresses the purpose of this laboratory exercise. ...
... Introduction ( Why the experiment is important? ) – State a hypothesis (an “if then” statement, may require multiple sentences) that is clear and appropriately addresses the purpose of this laboratory exercise. ...
Analysis of Whole-Body Branched-Chain Amino Acid Metabolism in
... acids (BCAAs – leucine, isoleucine and valine) are found in the serum of patients with early pancreatic cancers (Mayers, et al., 2014). Using a genetically engineered mouse model of PDAC (Bardeesy, et al., 2006) we used isotope-labeled diets to assess the sources of these plasma BCAA elevations and ...
... acids (BCAAs – leucine, isoleucine and valine) are found in the serum of patients with early pancreatic cancers (Mayers, et al., 2014). Using a genetically engineered mouse model of PDAC (Bardeesy, et al., 2006) we used isotope-labeled diets to assess the sources of these plasma BCAA elevations and ...
acetyl CoA - LSU School of Medicine
... A) Long chain fatty acids - activated by fatty acyl CoA synthase (thiokinase) (Fig. 15.6). Very important - 2 high energy bonds are used to activate a fatty acid. In typical reactions, ATP (2~) is converted to ADP (1~) and Pi (0~). In the thiokinase reaction, ATP (2~) is converted to AMP (0~) and PP ...
... A) Long chain fatty acids - activated by fatty acyl CoA synthase (thiokinase) (Fig. 15.6). Very important - 2 high energy bonds are used to activate a fatty acid. In typical reactions, ATP (2~) is converted to ADP (1~) and Pi (0~). In the thiokinase reaction, ATP (2~) is converted to AMP (0~) and PP ...
H - IS MU
... Polyol metabolism in diabetics • If the blood concentration of glucose is very high (e.g. in diabetes mellitus), large amount of glucose enter the cells • The polyol pathway produces glucitol. •It cannot pass efficiently through cytoplasmic membrane ...
... Polyol metabolism in diabetics • If the blood concentration of glucose is very high (e.g. in diabetes mellitus), large amount of glucose enter the cells • The polyol pathway produces glucitol. •It cannot pass efficiently through cytoplasmic membrane ...
29
... With the procedure, usually between 1 to 4 units of a person's own blood (autologos) are withdrawn, the plasma is removed and immediately reinfused, and the packed red cells are placed in frozen storage. To prevent a dramatic reduction in blood cell concentration, each unit of blood is withdrawn ove ...
... With the procedure, usually between 1 to 4 units of a person's own blood (autologos) are withdrawn, the plasma is removed and immediately reinfused, and the packed red cells are placed in frozen storage. To prevent a dramatic reduction in blood cell concentration, each unit of blood is withdrawn ove ...
AP BIOLOGY – CHAPTER 7 Cellular Respiration Outline
... 1) This series of reactions gives off CO2 and produces ATP. 2) Produces two immediate ATP molecules per glucose molecule. d. The electron transport system: 1) Series of carriers accepts electrons from glucose; electrons are passed from carrier to carrier until received by oxygen. 2) Electrons pass f ...
... 1) This series of reactions gives off CO2 and produces ATP. 2) Produces two immediate ATP molecules per glucose molecule. d. The electron transport system: 1) Series of carriers accepts electrons from glucose; electrons are passed from carrier to carrier until received by oxygen. 2) Electrons pass f ...
1.Lect .AADegradation
... throughout the body • The a.a. pool contains 100 gm a.as.50% of these a.as are in the form of glutamate & glutamine (Why?) • In contrast to the amount of protein in the body (about 12 Kg in 70 Kg man), the a.a. pool is small (only 100 gm) AA pool is not reserve !! There is not a specific protein res ...
... throughout the body • The a.a. pool contains 100 gm a.as.50% of these a.as are in the form of glutamate & glutamine (Why?) • In contrast to the amount of protein in the body (about 12 Kg in 70 Kg man), the a.a. pool is small (only 100 gm) AA pool is not reserve !! There is not a specific protein res ...
Citric Acid Cycle
... citric acid cycle, generating three NADH, one FADH2, and one ATP (by substrate-level phophorylation). • Intermediates of citric acid cycle are also used as biosynthetic precursors for many other biomolecules, including fatty acids, steroids, amino acids, heme, pyrimidines, and glucose. ...
... citric acid cycle, generating three NADH, one FADH2, and one ATP (by substrate-level phophorylation). • Intermediates of citric acid cycle are also used as biosynthetic precursors for many other biomolecules, including fatty acids, steroids, amino acids, heme, pyrimidines, and glucose. ...
WEB
... Glycosaminoglycans, etc. form Inclusions in lysosome Degradative enzymes in blood & urine ...
... Glycosaminoglycans, etc. form Inclusions in lysosome Degradative enzymes in blood & urine ...
L02_2002
... chain by up to 7 residues long (also donated by UDPG). Glycogen synthase can then attach glucose residues to this glycogen “primer”. Each molecule of glycogen is associated with ONE molecule each of glycogenin and glycogen synthase. ...
... chain by up to 7 residues long (also donated by UDPG). Glycogen synthase can then attach glucose residues to this glycogen “primer”. Each molecule of glycogen is associated with ONE molecule each of glycogenin and glycogen synthase. ...
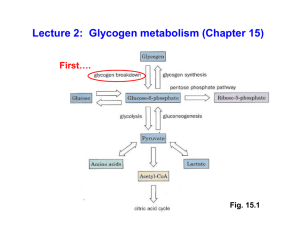
Lecture 2: Glycogen metabolism (Chapter 15)
... 2. Fatty acid residues cannot be metabolized anaerobically (that is, without oxygen). (If you want to burn fat while you are exercising, you must be able to breathe fairly easily.) 3. Animals cannot convert fat to glucose, so fat metabolism cannot maintain blood glucose levels. (Glucose is ”brain fo ...
... 2. Fatty acid residues cannot be metabolized anaerobically (that is, without oxygen). (If you want to burn fat while you are exercising, you must be able to breathe fairly easily.) 3. Animals cannot convert fat to glucose, so fat metabolism cannot maintain blood glucose levels. (Glucose is ”brain fo ...
File
... two G3P but 4 ATP are made while rearranging them into pyruvate; therefore, glycolysis has a net production of 2 ATP ...
... two G3P but 4 ATP are made while rearranging them into pyruvate; therefore, glycolysis has a net production of 2 ATP ...
Biochemistry II, Test One
... C. Transketolase and transaldolase link this pathway to gluconeogenesis. F D. It is more active in muscle cells than in fat-storage cells. F E. It interconverts trioses, tetroses, pentoses, hexoses, and heptoses. T 7. Which of the following statements are correct? The citric acid cycle (2 points) A. ...
... C. Transketolase and transaldolase link this pathway to gluconeogenesis. F D. It is more active in muscle cells than in fat-storage cells. F E. It interconverts trioses, tetroses, pentoses, hexoses, and heptoses. T 7. Which of the following statements are correct? The citric acid cycle (2 points) A. ...
3. CITRIC ACID CYCLE
... succinate thiokinase (succiny1CoA synthetase). High-energy phosphate (ATP) is synthesized at the substrate level because the release of free energy from the oxidative decarboxylation of α- ketoglutarate. The reaction requires GDP or IDP which is converted to GTP or ITP in the presence of inorganic p ...
... succinate thiokinase (succiny1CoA synthetase). High-energy phosphate (ATP) is synthesized at the substrate level because the release of free energy from the oxidative decarboxylation of α- ketoglutarate. The reaction requires GDP or IDP which is converted to GTP or ITP in the presence of inorganic p ...
BIOMOLECULES
... 39. α-Helix is a secondary structure of proteins formed by twisting of polypeptide chain into right handed screw like structures. Which type of interactions are responsible for making the α-helix structure stable? 40. Some enzymes are named after the reaction, where they are used. What name is given ...
... 39. α-Helix is a secondary structure of proteins formed by twisting of polypeptide chain into right handed screw like structures. Which type of interactions are responsible for making the α-helix structure stable? 40. Some enzymes are named after the reaction, where they are used. What name is given ...
Hormones of the Gut
... bladder to contract--cholecystokinin. 2. 1940s: Extract of duodenal mucosa stimulates pancreas to secrete enzymes--pancreozymin. 3. 1964-8: Purification of a single substance that stimulated both contraction of the gall bladder and pancreatic enzyme secretion--settled on one name: cholecystokinin (C ...
... bladder to contract--cholecystokinin. 2. 1940s: Extract of duodenal mucosa stimulates pancreas to secrete enzymes--pancreozymin. 3. 1964-8: Purification of a single substance that stimulated both contraction of the gall bladder and pancreatic enzyme secretion--settled on one name: cholecystokinin (C ...
Lecture 6
... • If oxygen is available, the 2 pyruvate will be broken down into 6 CO2 and ATP • Takes place in the matrix ...
... • If oxygen is available, the 2 pyruvate will be broken down into 6 CO2 and ATP • Takes place in the matrix ...
Study Questions for Chapter 1 – The Cell
... 4. When plotting the velocity (V) of an enzymatic reaction against the substrate concentration, one sees “saturable” kinetics. That is, at some substrate concentration, the enzyme is functioning at its maximal rate (Vmax) and cannot operate any faster. The substrate concentration that results in ...
... 4. When plotting the velocity (V) of an enzymatic reaction against the substrate concentration, one sees “saturable” kinetics. That is, at some substrate concentration, the enzyme is functioning at its maximal rate (Vmax) and cannot operate any faster. The substrate concentration that results in ...
Ketosis

Ketosis /kɨˈtoʊsɨs/ is a metabolic state where most of the body's energy supply comes from ketone bodies in the blood, in contrast to a state of glycolysis where blood glucose provides most of the energy. It is characterised by serum concentrations of ketone bodies over 0.5 millimolar, with low and stable levels of insulin and blood glucose. It is almost always generalized with hyperketonemia, that is, an elevated level of ketone bodies in the blood throughout the body. Ketone bodies are formed by ketogenesis when liver glycogen stores are depleted (or from metabolising medium-chain triglycerides). The main ketone bodies used for energy are acetoacetate and β-hydroxybutyrate, and the levels of ketone bodies are regulated mainly by insulin and glucagon. Most cells in the body can use both glucose and ketone bodies for fuel, and during ketosis, free fatty acids and glucose synthesis (gluconeogenesis) fuel the remainder.Longer-term ketosis may result from fasting or staying on a low-carbohydrate diet, and deliberately induced ketosis serves as a medical intervention for intractable epilepsy. In glycolysis, higher levels of insulin promote storage of body fat and block release of fat from adipose tissues, while in ketosis, fat reserves are readily released and consumed. For this reason, ketosis is sometimes referred to as the body's ""fat burning"" mode.