Lecture 20: Polyelectronic Atoms
... • For polyelectronic (i.e. real) atoms, a direct solution of the Schrodinger Equation is not possible. (Can’t solve the 3 body motion problem; Z12.61) • When we construct polyelectronic atoms, we use the hydrogen-atom orbital nomenclature to discuss in which orbitals the electrons reside. • This is ...
... • For polyelectronic (i.e. real) atoms, a direct solution of the Schrodinger Equation is not possible. (Can’t solve the 3 body motion problem; Z12.61) • When we construct polyelectronic atoms, we use the hydrogen-atom orbital nomenclature to discuss in which orbitals the electrons reside. • This is ...
Chemistry Week 04 - nchsdduncanchem1
... No two electrons in an atom have the same set of four quantum numbers. Hund's Rule: Electrons will enter empty orbitals of equal energy, when they are available. Quantum Chemistry: Describes the way atoms combine to form molecules and the way molecules interact with one another, using the rules of q ...
... No two electrons in an atom have the same set of four quantum numbers. Hund's Rule: Electrons will enter empty orbitals of equal energy, when they are available. Quantum Chemistry: Describes the way atoms combine to form molecules and the way molecules interact with one another, using the rules of q ...
CH7 handout is here.
... 8. Heisenberg uncertainty principle states that we cannot know exactly the position and velocity of an electron both at the same instant. Explain what we studied under ‘position’ and under ‘velocity’. What were the assumptions when studying ‘position’? “velocity”? ...
... 8. Heisenberg uncertainty principle states that we cannot know exactly the position and velocity of an electron both at the same instant. Explain what we studied under ‘position’ and under ‘velocity’. What were the assumptions when studying ‘position’? “velocity”? ...
Chapter 7 - Gordon State College
... • For n 2, the s- and p-orbitals are no longer degenerate because the electrons interact with each other. • Therefore, the Aufbau diagram looks slightly different for many-electron systems. ...
... • For n 2, the s- and p-orbitals are no longer degenerate because the electrons interact with each other. • Therefore, the Aufbau diagram looks slightly different for many-electron systems. ...
C:\Documents and Settings\Travis D. Fridgen\My Documents
... e) period 4 transition element that forms a 3+ diamagnetic ion. (Scandium) f) period 5 element that forms 3+ ion with a “pseudo-rare-gas” configuration. (Indium) g) whose ground state electron configuration is [Ne]3s23p2. (Silicon) h) ground state electron configuration is [Kr]5s24d6. (Ruthenium) i) ...
... e) period 4 transition element that forms a 3+ diamagnetic ion. (Scandium) f) period 5 element that forms 3+ ion with a “pseudo-rare-gas” configuration. (Indium) g) whose ground state electron configuration is [Ne]3s23p2. (Silicon) h) ground state electron configuration is [Kr]5s24d6. (Ruthenium) i) ...
Chemistry ~ Fall Final Review
... 4. The density of iron is 7.80 g/cm3. What is the mass of a 0.892 cm3 sample? 5. Define physical and chemical change. 6. Define element, atom, compound, molecule, mixture, solution. 7. Explain how to determine the # of protons, neutrons and electrons from given information about atomic mass, atomic ...
... 4. The density of iron is 7.80 g/cm3. What is the mass of a 0.892 cm3 sample? 5. Define physical and chemical change. 6. Define element, atom, compound, molecule, mixture, solution. 7. Explain how to determine the # of protons, neutrons and electrons from given information about atomic mass, atomic ...
Chapter 7
... how light acts as both a particle and as a wave. Atomic Spectroscopy was developed to explore the ...
... how light acts as both a particle and as a wave. Atomic Spectroscopy was developed to explore the ...
chapter 7: atomic structure and periodicity
... Wavelength (λ) - ____________________ between 2 successive crests or troughs Frequency (ν) - the number of _____________ (cycles) per second that pass a certain point. (unit: Speed (c) – speed of light = _______________________________ ...
... Wavelength (λ) - ____________________ between 2 successive crests or troughs Frequency (ν) - the number of _____________ (cycles) per second that pass a certain point. (unit: Speed (c) – speed of light = _______________________________ ...
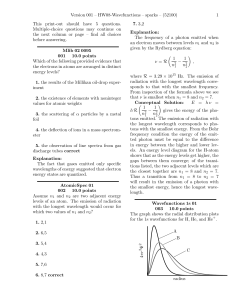
ATOMIC STRUCTURE NOTES n hcZ E ℜ
... General Periodic Trends – descending a group atomic radii increase, and with s & p blocks they decrease from left to right across period. Period 6 is different, due to lanthanide contraction. The 4f orbitals are being occupied by the lanthanides, and these have poor shielding properties. The repulsi ...
... General Periodic Trends – descending a group atomic radii increase, and with s & p blocks they decrease from left to right across period. Period 6 is different, due to lanthanide contraction. The 4f orbitals are being occupied by the lanthanides, and these have poor shielding properties. The repulsi ...
國立屏東教育大學95學年度研究所碩士班入學考試
... 1. If matter is uniform throughout, cannot be separated into other substances by physical processes, but can be decomposed into other substances by chemical processes, it is called a (an) __________. (A) heterogeneous mixture (B) element (C) homogeneous mixture (D) compound (E) mixture of elements 2 ...
... 1. If matter is uniform throughout, cannot be separated into other substances by physical processes, but can be decomposed into other substances by chemical processes, it is called a (an) __________. (A) heterogeneous mixture (B) element (C) homogeneous mixture (D) compound (E) mixture of elements 2 ...
Modern Physics
... the nucleus than r = ao (that is between r = 0 and r = ao). Verify this by calculating the relevant probabilities? ...
... the nucleus than r = ao (that is between r = 0 and r = ao). Verify this by calculating the relevant probabilities? ...
x 100 QUANTUM NUMBERS AND SYMBOLS
... 5. What type of orbital in an atom is designated by quantum numbers n=4, l =3, and ml =0? 6. A subshell in an atom has the values, n = 3, l =2. How many orbitals are there in this ...
... 5. What type of orbital in an atom is designated by quantum numbers n=4, l =3, and ml =0? 6. A subshell in an atom has the values, n = 3, l =2. How many orbitals are there in this ...
NAME PRACTICE: QUANTUM CONFIGURATIONS 1) Each of the
... ___20) The ground-state configuration for the atoms of a transition element ___21) The ground-state configuration of a negative ion of a halogen ___22) The ground-state configuration of a common ion of an alkaline earth element Use these answers for questions 23-25. (1) Heisenberg uncertainty princi ...
... ___20) The ground-state configuration for the atoms of a transition element ___21) The ground-state configuration of a negative ion of a halogen ___22) The ground-state configuration of a common ion of an alkaline earth element Use these answers for questions 23-25. (1) Heisenberg uncertainty princi ...
Problem-set-6
... 2) Rydberg states and their peculiar properties Consider a hydrogen atom in a state with principal quantum number n=45. These states with high-n quantum numbers are usually called Rydberg states. a) What is the binding energy of this state, calculated in eV and in units of cm-1. b) What is the absor ...
... 2) Rydberg states and their peculiar properties Consider a hydrogen atom in a state with principal quantum number n=45. These states with high-n quantum numbers are usually called Rydberg states. a) What is the binding energy of this state, calculated in eV and in units of cm-1. b) What is the absor ...
Modules to examine on the Arrangement of Electrons in Atoms website
... Modules to examine on the Arrangement of Electrons in Atoms website: (click to go directly to the different modules) EM Waves Evidence for EM Waves Catch the Wave Stadium Wave Electric Force Quantum Atom Spectral Lines Edvidence for Spectra Absorption Spectra Bohr's Atom Vibrating Charges rev. Adv B ...
... Modules to examine on the Arrangement of Electrons in Atoms website: (click to go directly to the different modules) EM Waves Evidence for EM Waves Catch the Wave Stadium Wave Electric Force Quantum Atom Spectral Lines Edvidence for Spectra Absorption Spectra Bohr's Atom Vibrating Charges rev. Adv B ...
Quantum Numbers, Orbitals, Electron Configurations, Periodic Trends
... How many significant figures are in each of the following numbers? a) 34.02 c) 10.50 e) 1.2340 × 107 b) 3300 d) 0.00342 f) 12340000 Convert the numbers is questions 1a – 1d into scientific notation. a) c) b) d) Convert the following numbers that are in scientific notation into decimal form. a) 1.234 ...
... How many significant figures are in each of the following numbers? a) 34.02 c) 10.50 e) 1.2340 × 107 b) 3300 d) 0.00342 f) 12340000 Convert the numbers is questions 1a – 1d into scientific notation. a) c) b) d) Convert the following numbers that are in scientific notation into decimal form. a) 1.234 ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.