* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Electron Configuration
Ferromagnetism wikipedia , lookup
Molecular orbital wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Chemical bond wikipedia , lookup
Particle in a box wikipedia , lookup
Bremsstrahlung wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Double-slit experiment wikipedia , lookup
Astronomical spectroscopy wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
Hydrogen atom wikipedia , lookup
Tight binding wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Matter wave wikipedia , lookup
Atomic orbital wikipedia , lookup
Atomic theory wikipedia , lookup
Wave–particle duality wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
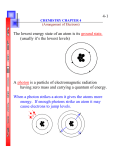
Electron Configuration Chapter 5. Sec 5.1 Electrons in atoms 1. In the 1900s scientists observed that certain elements emitted visible light when heated in a flame. The analysis of that flame revealed that the chemical behavior is related to the arrangement of the electrons in its atom. 2. Scientists also observed that light behave somehow like the electrons. Understanding light behavior helped them in explaining how electrons behave. 3. What is that scientists discovered regarding to the element’s chemical behavior? Scientists discovered that an element’s chemical behavior is related to the arrangement of the electrons in its atoms. 4. How does light travel? In waves in a pattern called electromagnetic radiation (through space) 5. What is electromagnetic radiation? It is a form of energy that exhibits wavelike behavior as it travels through space 6. If light travels in waves, which are the characteristics of waves? Waves have four characteristics: amplitude, wavelength, frequency and speed. 7. How is frequency measured? Frequency tells you how fast a wave moves in a second. 8. How is wavelength measured? It is the distance between equivalent points on a continuous wave. Crest to crest or trough to trough 9. How is amplitude measured? It is the wave’s height from the origin to a crest or trough 10. How fast electromagnetic waves travel including light? Light moves at a speed of 3 x 108 m/s 11. Light differ from other types of waves because it travels at a constant speed C= wavelength (λ) x frequency (υ) (formula to calculate speed of light in vacuum). 12. How are wavelength and frequency related? Inversely, Long wavelength low frequency; short wavelength high frequency 13. What is the electromagnetic spectrum? Since waves travel at different speeds and therefore have different frequencies and wavelengths. The electromagnetic spectrum encompasses all the forms of electromagnetic radiation. 14. What is a quantum? A quantum is the minimum amount of energy that can be gained or lost 15. Who came up with that concept? Max Plank 16. Plank was able to predict how the emission of different colors of light was produced when heating objects. As molecules were agitated they shifted in speed and in wavelength producing different colors. In other words, he found the relationship that exists between the frequency and wavelength of a particular type of radiation to the energy it carries. 17. This relationship was expressed in what is known as the Plank’s constant Energy = Plank’s constant x frequency (E=hv) v= Plank’s constant (6.62 x 10-34 J/sec) 18. His findings were the base knowledge that led Einstein to discover that energy is carried by photons. Each photon or particles of light can only carry certain quanta (amount of energy). This explanation aid in understanding the effects of different kinds of electromagnetic radiation. 19. Einstein used Planck’s equation to explain what the photoelectric effect was. In the photoelectric effect, electrons are ejected from the surface of a metal when light shines on the metal. He then proposed that light consists of quantifiable energy in the form of photons. This helped in understanding the effects of the different waves produced in the electromagnetic spectrum. High frequency energy or high quantified photons are damaging. 20. What is a line spectrum? It is a portion of the spectrum that contains certain colors or wavelengths. Some elements emit light when vaporized in a flame. The line spectrum is also known as the atomic emission spectrum (kind of a finger print of an element). Section 5.2 Quantum Theory 21. Bohr Model. Bohr postulated that to get spectral lines, the energy of the electron must be quantized (measured). Using Rutherford’s model, he labeled each energy level with a quantum number called “n.” The lowest energy level known as the ground state had a quantum number of one, meaning n= 1. This is the energy level closest to the nucleus. When an electron absorbs energy it is said to be in an excited state and moves to a higher-energy levels called n=2, 3, 4-7 etc. 22. Louis de Broglie analyzed Bohr’s ideas and suggested that electrons had wavelike motion and that electrons move in certain wavelengths frequencies and energies. He created a formula that calculates the probability of finding the electron ( λ= h/mv) Page 150. 23. After analyzing Broglies and Bohr’s ideas, Werner Heisenberg stated that it is impossible to know precisely the velocity and position of a particle at the same time, therefore difficult to know the exact position of the electron. 24. The Heisenberg uncertainty principle states that it is fundamentally impossible to know precisely both the velocity and position of a particle at the same time. 25. Schodinger, an Australian physicist created a model where electrons are treated as waves called the wave mechanical model of the atom or the quantum mechanical model of the atom. As a result instead of the circular orbits a 3-D region around the cloud was called the atomic orbital. An atomic orbital is the region around the nucleus of an atom where an electron is likely to be found 26. The cloud is most dense where the probability of finding the electron is higher and that is called the electron density 27. The principal quantum number “n” indicates the relative size and energy of atomic orbitals. As n increases the orbital becomes larger. According to Bohr there 7 energy levels (shells). 28. Principal energy levels contain energy sublevels named s, p, d, f; each sublevel have different shapes. S orbitals have a spherical shape, p orbitals have a dumbbell shape and d, or f orbitals have different shapes and orientations. Section 5.3 Electron Configuration 29. Electron spin: electrons have particular energies and they spin on their own axis. The spin can be clockwise or counter clock wise. The spin of the electron creates a magnetic field. 30. In 1925 Wolfgang Pauli stated the importance of the electron spin in determining how electrons are arranged. Pauli Exclusion Principle stated that a maximum of two electrons may occupy a single atomic orbital. 31. Hund’s rule states that negatively charged electrons repel each other, so electrons with the same spin must occupy each equal-energy orbital before electrons with opposite spin occupy the same orbital (page 157) Example: 1s1 2s2 32. Orbital diagrams Orbitals can be represented by boxes, each box is labeled with the principal quantum number n= 1, 2, etc. Each box should have the sublevels particular to each energy level s, p, d, f, these boxes are to be filled with arrows up an down representing the electrons found in each level (p. 158). 33. Electron configuration notation is a shorthand method for describing electron arrangement using the Aufbau principle. The Aufbau principle states that each electron occupies the lowest energy orbital available 34. Noble-gas notation is a form of representing electron configurations of noble gases using bracketed symbols (p. 159).