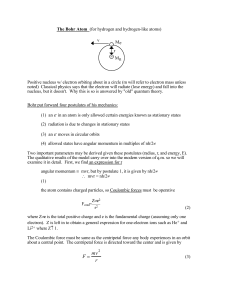
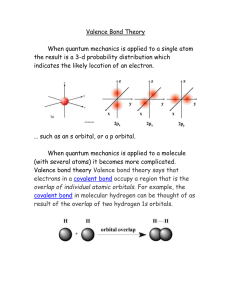
APCh7MB
... Smallest possible uncertainty = h/4π (h/4π)2 = probability distribution Radius of the sphere encloses 90% of the total electron probability ...
... Smallest possible uncertainty = h/4π (h/4π)2 = probability distribution Radius of the sphere encloses 90% of the total electron probability ...
quantum numbers - Cloudfront.net
... Shape of Electron Cloud (l) Also known as sublevel or subshell Indicates the shape of the orbital within a shell Only integer values between 0 and n-1 are allowed Affects orbital energies (bigger l = higher energy) All electrons in an atom with the same value of l are said to belong to the same subs ...
... Shape of Electron Cloud (l) Also known as sublevel or subshell Indicates the shape of the orbital within a shell Only integer values between 0 and n-1 are allowed Affects orbital energies (bigger l = higher energy) All electrons in an atom with the same value of l are said to belong to the same subs ...
Quantum Mechanics Problem Set
... (a) The uncertainty principle states that there is a limit to how precisely we can simultaneously know the position and momentum (a quantity relates to energy) of an electron. The Bohr model states that electrons move about the nucleus in precisely circular orbits of known radius and energy. This vi ...
... (a) The uncertainty principle states that there is a limit to how precisely we can simultaneously know the position and momentum (a quantity relates to energy) of an electron. The Bohr model states that electrons move about the nucleus in precisely circular orbits of known radius and energy. This vi ...
CHEMISTRY
... The nature of most atoms is that they are LONELY and sometimes AGGRESSIVE! Most atoms team up with or overtake other atoms in an attempt to get the “right” number of electrons. This is how molecules are formed. Only the NOBLE GASSES can exist on their own. ATOMS will switch partners when provoked. T ...
... The nature of most atoms is that they are LONELY and sometimes AGGRESSIVE! Most atoms team up with or overtake other atoms in an attempt to get the “right” number of electrons. This is how molecules are formed. Only the NOBLE GASSES can exist on their own. ATOMS will switch partners when provoked. T ...
Unit 3 – Quantum Mechanical Model of the Atom
... • The light corresponds to a certain wavelength & certain color. ...
... • The light corresponds to a certain wavelength & certain color. ...
Chemical Bonding
... Atoms of different elements can join together to form new substances. A substance which is made up of two or more different types of atoms is known as a compound. One way this can occur is for atoms to form ions. ...
... Atoms of different elements can join together to form new substances. A substance which is made up of two or more different types of atoms is known as a compound. One way this can occur is for atoms to form ions. ...
(Bohr Model And X-Rays) Part-1
... model had. This model introduced quantization principal and this model is regarded as first quantum mechanical model. We will explore in detail, this structure of atom. We will also look into X - ray formation which is a converse process of photoclectric effect and ...
... model had. This model introduced quantization principal and this model is regarded as first quantum mechanical model. We will explore in detail, this structure of atom. We will also look into X - ray formation which is a converse process of photoclectric effect and ...
Chemistry - nyostrander.us
... element return to the ground state. This emitted energy can be used to determine the (1) mass of the sample (3) identity of the element (2) volume of the sample (4) number of moles of the element 4. According to the wave-mechanical model, an orbital is defined as the (1) circular path for electrons ...
... element return to the ground state. This emitted energy can be used to determine the (1) mass of the sample (3) identity of the element (2) volume of the sample (4) number of moles of the element 4. According to the wave-mechanical model, an orbital is defined as the (1) circular path for electrons ...
Orbitals and energy levels
... to move an electron from one energy level to another energy level. ...
... to move an electron from one energy level to another energy level. ...
The Quantum Mechanical Model of the Atom
... • E is the total energy of the atom (the sum of the potential energy due to the attraction between the proton and electron and the kinetic energy of the moving electron) • When the equation is analyzed, many solutions are found. – Each solution consists of a wave function that is characterized by a ...
... • E is the total energy of the atom (the sum of the potential energy due to the attraction between the proton and electron and the kinetic energy of the moving electron) • When the equation is analyzed, many solutions are found. – Each solution consists of a wave function that is characterized by a ...
Many-Electron Atoms Thornton and Rex, Ch. 8
... Many-Electron Atoms For many-electron atoms there is now orbit-orbit and spin-spin interactions, in addition to spin-orbit interactions. Consider simplest case of 2 electrons with L1, S1 and L2, S2. Only “good” quantum number is ...
... Many-Electron Atoms For many-electron atoms there is now orbit-orbit and spin-spin interactions, in addition to spin-orbit interactions. Consider simplest case of 2 electrons with L1, S1 and L2, S2. Only “good” quantum number is ...
Many-Electron Atoms Thornton and Rex, Ch. 8
... Many-Electron Atoms For many-electron atoms there is now orbit-orbit and spin-spin interactions, in addition to spin-orbit interactions. Consider simplest case of 2 electrons with L1, S1 and L2, S2. Only “good” quantum number is ...
... Many-Electron Atoms For many-electron atoms there is now orbit-orbit and spin-spin interactions, in addition to spin-orbit interactions. Consider simplest case of 2 electrons with L1, S1 and L2, S2. Only “good” quantum number is ...
PHY140Y 32 The Pauli Exclusion Principle
... observed was that multi-electron atoms seemed to have periodic properties. As you added electrons to the atom, one found that the electrons appeared to occupy increasingly higher-energy “shells,” with two electrons per shell. This led to the following principle: You could not have two or more electr ...
... observed was that multi-electron atoms seemed to have periodic properties. As you added electrons to the atom, one found that the electrons appeared to occupy increasingly higher-energy “shells,” with two electrons per shell. This led to the following principle: You could not have two or more electr ...
Atomic Theory - WaylandHighSchoolChemistry
... in different energy levels, or orbits around the nucleus. • Electrons can change energy levels; higher energy levels are further from the nucleus. electron ...
... in different energy levels, or orbits around the nucleus. • Electrons can change energy levels; higher energy levels are further from the nucleus. electron ...
Electron configuration
In atomic physics and quantum chemistry, the electron configuration is the distribution of electrons of an atom or molecule (or other physical structure) in atomic or molecular orbitals. For example, the electron configuration of the neon atom is 1s2 2s2 2p6.Electronic configurations describe electrons as each moving independently in an orbital, in an average field created by all other orbitals. Mathematically, configurations are described by Slater determinants or configuration state functions.According to the laws of quantum mechanics, for systems with only one electron, an energy is associated with each electron configuration and, upon certain conditions, electrons are able to move from one configuration to another by the emission or absorption of a quantum of energy, in the form of a photon.Knowledge of the electron configuration of different atoms is useful in understanding the structure of the periodic table of elements. The concept is also useful for describing the chemical bonds that hold atoms together. In bulk materials, this same idea helps explain the peculiar properties of lasers and semiconductors.