ATOMIC STRUCTURE Chapter 7
... From Bohr model to Quantum mechanics Bohr’s theory was a great accomplishment and radically changed our view of matter. But problems existed with Bohr theory — – theory only successful for the H atom. ...
... From Bohr model to Quantum mechanics Bohr’s theory was a great accomplishment and radically changed our view of matter. But problems existed with Bohr theory — – theory only successful for the H atom. ...
Chemistry Science Notebook
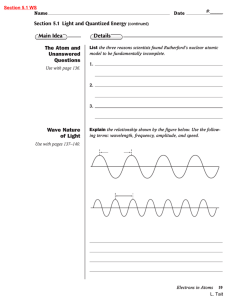
... List the three reasons scientists found Rutherford’s nuclear atomic model to be fundamentally incomplete. ...
... List the three reasons scientists found Rutherford’s nuclear atomic model to be fundamentally incomplete. ...
atomsagain
... •If you ignore the interaction of electrons, then 3s = 3p = 3d •In fact, the inner electrons are so close to the nucleus, the nuclear charge looks like it is reduced •Unless the “outer” electron penetrates inside a bit •Behavior near the nucleus is governed by l •Small l values can go near nucleus • ...
... •If you ignore the interaction of electrons, then 3s = 3p = 3d •In fact, the inner electrons are so close to the nucleus, the nuclear charge looks like it is reduced •Unless the “outer” electron penetrates inside a bit •Behavior near the nucleus is governed by l •Small l values can go near nucleus • ...
CH 4 SEC 2: Book Notes
... ○ Electrons are both particles and waves. ○ Heisenberg’s idea involved the detection of electrons. Electrons are detected by their interaction with photons. ○ Because photons have about the same energy as electrons, any attempt to locate a specific electron with a photon knocks the electron off its ...
... ○ Electrons are both particles and waves. ○ Heisenberg’s idea involved the detection of electrons. Electrons are detected by their interaction with photons. ○ Because photons have about the same energy as electrons, any attempt to locate a specific electron with a photon knocks the electron off its ...
Chapter 4
... We can show this on different line spectrum or lineemission spectrum. Each of the colored lines is produced by light of a different wavelength. ...
... We can show this on different line spectrum or lineemission spectrum. Each of the colored lines is produced by light of a different wavelength. ...
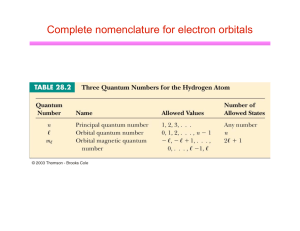
Complete nomenclature for electron orbitals
... What happens? Some of the electrons can accelerate and gain energy. This is possible because the conduction band is close in energy to the valence band and there are empty energy states to jump into. This can’t happen with insulators where there is too large of an energy gap between. ...
... What happens? Some of the electrons can accelerate and gain energy. This is possible because the conduction band is close in energy to the valence band and there are empty energy states to jump into. This can’t happen with insulators where there is too large of an energy gap between. ...
Atomic Structure and Electron Configurations Multiple Choice PSI
... C. Angular quantum number (l) which describes the shape of an electron’s orbital D. Magnetic quantum number (ml) which describes the orbitals orientation in space 7. The Heisenberg Uncertainty Principle A. assumes that the electrons take positions predicted by Bohr's theory. B. states that the posit ...
... C. Angular quantum number (l) which describes the shape of an electron’s orbital D. Magnetic quantum number (ml) which describes the orbitals orientation in space 7. The Heisenberg Uncertainty Principle A. assumes that the electrons take positions predicted by Bohr's theory. B. states that the posit ...
Document
... From Bohr model to Quantum mechanics Bohr’s theory was a great accomplishment and radically changed our view of matter. But problems existed with Bohr theory — – theory only successful for the H atom. ...
... From Bohr model to Quantum mechanics Bohr’s theory was a great accomplishment and radically changed our view of matter. But problems existed with Bohr theory — – theory only successful for the H atom. ...
The Quantum Atom (section 18)
... electromagnetic radiation and lose energy. Why does the electron not do this? Excited gases emit line spectra: light at certain characteristic frequencies, not continuous spectra. They also absorb light only at these frequencies. Why? ...
... electromagnetic radiation and lose energy. Why does the electron not do this? Excited gases emit line spectra: light at certain characteristic frequencies, not continuous spectra. They also absorb light only at these frequencies. Why? ...
Lecture 9
... Let’s do an experiment: Look at the discharge lamps through the diffraction glasses. They work just like a prism and break up light into its components. Notice the dark spots between the “lines” of the different colors. The number and positions of the lines are the unique signature of the elements. ...
... Let’s do an experiment: Look at the discharge lamps through the diffraction glasses. They work just like a prism and break up light into its components. Notice the dark spots between the “lines” of the different colors. The number and positions of the lines are the unique signature of the elements. ...
Ch 6 Outline
... A laser used in eye surgery to fuse detached retinas produces radiation with a wavelength of 640.0 nm. Calculate the frequency of this radiation. ...
... A laser used in eye surgery to fuse detached retinas produces radiation with a wavelength of 640.0 nm. Calculate the frequency of this radiation. ...
Electron Configurations
... Principle we can not know the exact position and motion of electrons with complete certainty. • We can only describe the probable locations of electrons. • We will describe the location of electrons when the atom is at its lowest energy . ...
... Principle we can not know the exact position and motion of electrons with complete certainty. • We can only describe the probable locations of electrons. • We will describe the location of electrons when the atom is at its lowest energy . ...
Chem 1st Sem Rev Ch2
... d. planetary model of the atom, electrons move around the nucleus like planets around sun. e. plum pudding model of the atom: atom looks like chocolate chip cookie f. gold foil experiment – atoms have a dense core called nucleus g. he gave the wave equation – mathematical description of location of ...
... d. planetary model of the atom, electrons move around the nucleus like planets around sun. e. plum pudding model of the atom: atom looks like chocolate chip cookie f. gold foil experiment – atoms have a dense core called nucleus g. he gave the wave equation – mathematical description of location of ...
Quantum Theory of Atoms
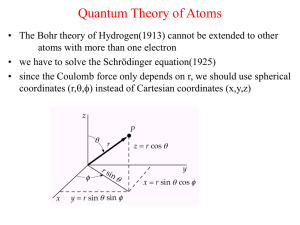
... Quantum Theory of Atoms • The Bohr theory of Hydrogen(1913) cannot be extended to other atoms with more than one electron • we have to solve the Schrödinger equation(1925) • since the Coulomb force only depends on r, we should use spherical coordinates (r,,) instead of Cartesian coordinates (x,y,z ...
... Quantum Theory of Atoms • The Bohr theory of Hydrogen(1913) cannot be extended to other atoms with more than one electron • we have to solve the Schrödinger equation(1925) • since the Coulomb force only depends on r, we should use spherical coordinates (r,,) instead of Cartesian coordinates (x,y,z ...
Atomic Radii Answers File
... charge has not changed. However, now the nucleus is attracting one less electron so the remaining ones are pulled in closer. When an atom gains an electron to form a negative ion, the nuclear charge has not changed. However, now the nucleus is attracting one more electron so they are not pulled as s ...
... charge has not changed. However, now the nucleus is attracting one less electron so the remaining ones are pulled in closer. When an atom gains an electron to form a negative ion, the nuclear charge has not changed. However, now the nucleus is attracting one more electron so they are not pulled as s ...
Chemistry Notes
... I. The Chemistry of matter A. All matter in the universe is composed of atoms. B. Parts of an atom 1. Electron Shell – The outer part of an atom. This is where the electrons are found. a) Electrons – Negatively charged particles found in the electron shell. Electrons orbit the nucleus. 2. Nucleus – ...
... I. The Chemistry of matter A. All matter in the universe is composed of atoms. B. Parts of an atom 1. Electron Shell – The outer part of an atom. This is where the electrons are found. a) Electrons – Negatively charged particles found in the electron shell. Electrons orbit the nucleus. 2. Nucleus – ...
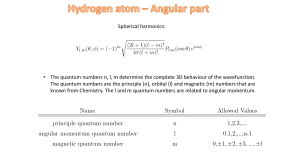
Spherical harmonics: • The quantum numbers n, l, m determine the
... The quantum numbers are the principle (n), orbital (l) and magnetic (m) numbers that are known from Chemistry. The l and m quantum numbers are related to angular momentum. ...
... The quantum numbers are the principle (n), orbital (l) and magnetic (m) numbers that are known from Chemistry. The l and m quantum numbers are related to angular momentum. ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.