Name
... same as the emission spectrum of another element. 16. Only electrons moving from energy levels lose energy and emit light. ...
... same as the emission spectrum of another element. 16. Only electrons moving from energy levels lose energy and emit light. ...
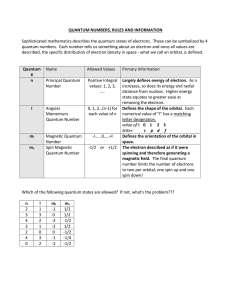
Quantum Numbers
... Only 2 electrons are allowed in each orbital Each with an opposite spin. Given the following quantum numbers, describe the “probable” location of the electron. n, l, m, s, ...
... Only 2 electrons are allowed in each orbital Each with an opposite spin. Given the following quantum numbers, describe the “probable” location of the electron. n, l, m, s, ...
ATOMIC STRUCTURE NOTES n hcZ E ℜ
... General Periodic Trends – descending a group atomic radii increase, and with s & p blocks they decrease from left to right across period. Period 6 is different, due to lanthanide contraction. The 4f orbitals are being occupied by the lanthanides, and these have poor shielding properties. The repulsi ...
... General Periodic Trends – descending a group atomic radii increase, and with s & p blocks they decrease from left to right across period. Period 6 is different, due to lanthanide contraction. The 4f orbitals are being occupied by the lanthanides, and these have poor shielding properties. The repulsi ...
Chapter 7_01042016
... • In hydrogen 1s orbital radius of the sphere that encloses 90% of the total electron probability. ...
... • In hydrogen 1s orbital radius of the sphere that encloses 90% of the total electron probability. ...
Quantum Number Table
... Largely defines energy of electron. As n increases, so does its energy and radial distance from nucleus. Higher energy state equates to greater ease at removing the electron. Defines the shape of the orbital. Each numerical value of "l" has a matching letter designation. value of l: 0 1 2 3 letter: ...
... Largely defines energy of electron. As n increases, so does its energy and radial distance from nucleus. Higher energy state equates to greater ease at removing the electron. Defines the shape of the orbital. Each numerical value of "l" has a matching letter designation. value of l: 0 1 2 3 letter: ...
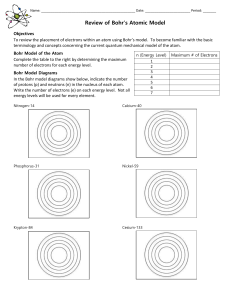
Name: ______ Date: Period: ______ Review of Bohr`s Atomic Model
... In the Bohr model diagrams show below, indicate the number of protons (p) and neutrons (n) in the nucleus of each atom. Write the number of electrons (e) on each energy level. Not all energy levels will be used for every element. ...
... In the Bohr model diagrams show below, indicate the number of protons (p) and neutrons (n) in the nucleus of each atom. Write the number of electrons (e) on each energy level. Not all energy levels will be used for every element. ...
Atomic Emission Spectra and Quantum mechanical Model
... cars, baseball, marbles • In quantum mechanics, matter moves like waves and it ...
... cars, baseball, marbles • In quantum mechanics, matter moves like waves and it ...
Quantum Mechanical Model
... experiment. Atom was made of dense nucleus with + charge. Nucleus was surrounded by empty space and electrons. PROBLEM: The trouble with Rutherford’s model is that opposites attract. Why didn’t the electrons collapse into the nucleus? ...
... experiment. Atom was made of dense nucleus with + charge. Nucleus was surrounded by empty space and electrons. PROBLEM: The trouble with Rutherford’s model is that opposites attract. Why didn’t the electrons collapse into the nucleus? ...
CHAPTER 5
... 2. An electron may move from one discrete energy level (orbit) to another, but to do so energy is emitted or absorbed 3. An electron moves in a spherical orbit around the nucleus -If e- are in quantized energy states, then ∆E of states can have only certain values -This explains sharp line spectra ( ...
... 2. An electron may move from one discrete energy level (orbit) to another, but to do so energy is emitted or absorbed 3. An electron moves in a spherical orbit around the nucleus -If e- are in quantized energy states, then ∆E of states can have only certain values -This explains sharp line spectra ( ...
Essential Question: What is the current model of the atom? How
... Frequency Bohr model Energy levels ground state Photoelectric effect Azimuthal Q# Magnetic Q# Quantum model Electron cloud Pauli Exclusion Prin Electron configuration Orbital S orbital f orbitals sublevels Atomic mass Mass number Neutrons Electrons ...
... Frequency Bohr model Energy levels ground state Photoelectric effect Azimuthal Q# Magnetic Q# Quantum model Electron cloud Pauli Exclusion Prin Electron configuration Orbital S orbital f orbitals sublevels Atomic mass Mass number Neutrons Electrons ...
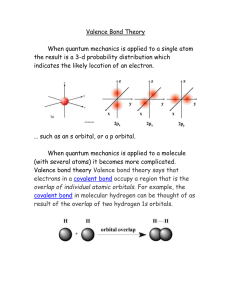
Valence Bond Theory
... When quantum mechanics is applied to a molecule (with several atoms) it becomes more complicated. Valence bond theory Valence bond theory says that electrons in a covalent bond occupy a region that is the overlap of individual atomic orbitals. For example, the covalent bond in molecular hydrogen can ...
... When quantum mechanics is applied to a molecule (with several atoms) it becomes more complicated. Valence bond theory Valence bond theory says that electrons in a covalent bond occupy a region that is the overlap of individual atomic orbitals. For example, the covalent bond in molecular hydrogen can ...
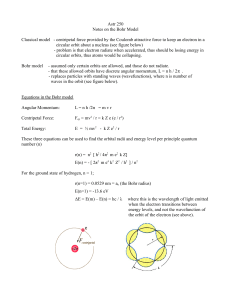
Astr 250 Notes on the Bohr Model Classical model
... Astr 250 Notes on the Bohr Model Classical model - centripetal force provided by the Coulomb attractive force to keep an electron in a circular orbit about a nucleus (see figure below) - problem is that electron radiate when accelerated, thus should be losing energy in circular orbits, thus atoms wo ...
... Astr 250 Notes on the Bohr Model Classical model - centripetal force provided by the Coulomb attractive force to keep an electron in a circular orbit about a nucleus (see figure below) - problem is that electron radiate when accelerated, thus should be losing energy in circular orbits, thus atoms wo ...
Chem 101A Exam 4 Concepts Chapter 7 – Modern Atomic Theory
... Chem 101A Exam 4 Concepts Chapter 7 – Modern Atomic Theory Use formulas that relate energy of photon, frequency, wavelength, speed of light, and the Rydberg Equation Notable scientists and their contributions: Rutherford, Bohr, Planc, de Broglie, Heisenberg, Schrödinger. The four Quantum ...
... Chem 101A Exam 4 Concepts Chapter 7 – Modern Atomic Theory Use formulas that relate energy of photon, frequency, wavelength, speed of light, and the Rydberg Equation Notable scientists and their contributions: Rutherford, Bohr, Planc, de Broglie, Heisenberg, Schrödinger. The four Quantum ...
7.4 The Wavelike properties of the Electron Models of
... • The solutions for the wavefunction, Ψ , in the H atom are called atomic orbitals • Born’s interpretation of the wavefunction – the probability to find the electron at a certain point (x, y, z) in space is proportional to the square of the wave function, Ψ 2, in this point • The atomic orbitals (Ψ ...
... • The solutions for the wavefunction, Ψ , in the H atom are called atomic orbitals • Born’s interpretation of the wavefunction – the probability to find the electron at a certain point (x, y, z) in space is proportional to the square of the wave function, Ψ 2, in this point • The atomic orbitals (Ψ ...
Multi-electron atoms have interactions between electrons, not just
... and nucleus! - The additional interactions in multi-electron atoms introduced added complexity to the model of the atom! Bohr's model was too simple. - Improvements in Bohr's model came from treating electrons as WAVES. de Broglie relationship ...
... and nucleus! - The additional interactions in multi-electron atoms introduced added complexity to the model of the atom! Bohr's model was too simple. - Improvements in Bohr's model came from treating electrons as WAVES. de Broglie relationship ...
Electronic Structure of Atoms (i.e., Quantum Mechanics)
... Carbon: Contains many more emission lines as compared to H. Why? ...
... Carbon: Contains many more emission lines as compared to H. Why? ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.