Basic Chemistry Notes II
... 3. Can be found by subtracting the atomic number from the atomic weight C. Electrons 1. Found outside of nucleus in “shells” 2. Have a negative charge 3. Valence electrons – outermost electron shell. Most important electrons because they determine bonding/reactivity of atom. ...
... 3. Can be found by subtracting the atomic number from the atomic weight C. Electrons 1. Found outside of nucleus in “shells” 2. Have a negative charge 3. Valence electrons – outermost electron shell. Most important electrons because they determine bonding/reactivity of atom. ...
Atomic Theory Study Guide - Reading Community Schools
... atom has a unique quantum number, which describes its probable position and energy. ...
... atom has a unique quantum number, which describes its probable position and energy. ...
Chapter 4
... not as particles, but more as waves (like light waves) which can gain or lose energy. But they can’t gain or lose just any amount of energy. They gain or lose a “quantum” of energy. A quantum is just an amount of energy that the electron needs to gain (or lose) to move to the next energy level. In ...
... not as particles, but more as waves (like light waves) which can gain or lose energy. But they can’t gain or lose just any amount of energy. They gain or lose a “quantum” of energy. A quantum is just an amount of energy that the electron needs to gain (or lose) to move to the next energy level. In ...
Lewis
... To understand the formation and structure of molecular compounds, first one has to learn, recognize, use, count, take into account: • the periodic table with groups and periods, • the number of electrons and valence electrons (i.e. count electrons), (2 (K), 8 (L) = 2 + 6, 18 (M) = 2 + 6 + 10, 32 (N) ...
... To understand the formation and structure of molecular compounds, first one has to learn, recognize, use, count, take into account: • the periodic table with groups and periods, • the number of electrons and valence electrons (i.e. count electrons), (2 (K), 8 (L) = 2 + 6, 18 (M) = 2 + 6 + 10, 32 (N) ...
Thursday, 1/29/09 - Liberty Union High School District
... Light as a Wave & Particle •Einstein’s radical idea! •Dual wave-particle nature of light •Called particles of light “photons” (no mass) •Explained photoelectric effect (photon must have minimum energy) •Light is absorbed only in whole numbers of photons ...
... Light as a Wave & Particle •Einstein’s radical idea! •Dual wave-particle nature of light •Called particles of light “photons” (no mass) •Explained photoelectric effect (photon must have minimum energy) •Light is absorbed only in whole numbers of photons ...
Electrons in the Atom
... not as particles, but more as waves (like light waves) which can gain or lose energy. But they can’t gain or lose just any amount of energy. They gain or lose a “quantum” of energy. A quantum is just an amount of energy that the electron needs to gain (or lose) to move to the next energy level. In ...
... not as particles, but more as waves (like light waves) which can gain or lose energy. But they can’t gain or lose just any amount of energy. They gain or lose a “quantum” of energy. A quantum is just an amount of energy that the electron needs to gain (or lose) to move to the next energy level. In ...
Quantum Numbe
... 1. Electrons have a dual nature – they can be both particles and waves - Bohr: an electron should be treated as a particle - Schroedinger: an electron should be treated as a wave ...
... 1. Electrons have a dual nature – they can be both particles and waves - Bohr: an electron should be treated as a particle - Schroedinger: an electron should be treated as a wave ...
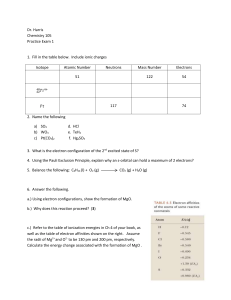
Dr. Harris Chemistry 105 Practice Exam 1 Isotope Atomic Number
... 14. A laser emits 200mJ of energy per hour. Given that the wavelength of the photons in the beam is 300 nm, and assuming that the emission rate is constant, how many photons are emitted per minute? ...
... 14. A laser emits 200mJ of energy per hour. Given that the wavelength of the photons in the beam is 300 nm, and assuming that the emission rate is constant, how many photons are emitted per minute? ...
Bohr Model and Quantum Model
... may know the location of an electron or the velocity of electron but you may not know both simultaneously ...
... may know the location of an electron or the velocity of electron but you may not know both simultaneously ...
Unit 3 Study Guide
... balancing oil drop in an electric field. Determines charge on an electron, and therefore mass of electron. Radioactive source of heavy positively charged alpha particles shot at very thin gold foil. Most go through, but some are highly deflected. When neon light is passed through a prism, you get li ...
... balancing oil drop in an electric field. Determines charge on an electron, and therefore mass of electron. Radioactive source of heavy positively charged alpha particles shot at very thin gold foil. Most go through, but some are highly deflected. When neon light is passed through a prism, you get li ...
Arrangement of Electrons in Atoms
... cannot know the instantaneous position and velocity of an electron (or any other particle) x – represents position p – represents momentum (velocity multiplied by mass) - represents a constant Δ – in this case, delta represents the uncertainty. ...
... cannot know the instantaneous position and velocity of an electron (or any other particle) x – represents position p – represents momentum (velocity multiplied by mass) - represents a constant Δ – in this case, delta represents the uncertainty. ...
Chapter 4-2 The Quantum Model of the Atom
... Heisenberg uncertainty principle laid the foundation for the modern quantum theory. Quantum theory describes mathematically the wave properties of electrons and other very small particles. ...
... Heisenberg uncertainty principle laid the foundation for the modern quantum theory. Quantum theory describes mathematically the wave properties of electrons and other very small particles. ...
Honors Chemistry First Marking Period Review Sheet
... I can apply the Heisenberg uncertainty principle: It is impossible to determine both the position and the momentum of an electron at the same time. For this reason, only the probability of the electron being within a given region of space (an “orbital”) can be calculated. I can apply the Pauli exclu ...
... I can apply the Heisenberg uncertainty principle: It is impossible to determine both the position and the momentum of an electron at the same time. For this reason, only the probability of the electron being within a given region of space (an “orbital”) can be calculated. I can apply the Pauli exclu ...
Atomic Structure Notes
... where n is an integer, h is Planck’s constant and ν is the frequency of the electromagnetic radiation absorbed or emitted. 2. Energy is in fact quantized and can only occur in discrete units of size hv. Each of these small "packets" of energy is called a quantum (or a photon when we are talking abou ...
... where n is an integer, h is Planck’s constant and ν is the frequency of the electromagnetic radiation absorbed or emitted. 2. Energy is in fact quantized and can only occur in discrete units of size hv. Each of these small "packets" of energy is called a quantum (or a photon when we are talking abou ...
Discovery of the Electron, Models & Theories
... Heisenberg also went off of Bohr’s model and elaborated more into quantum mechanics to explain the wave motion of electrons instead of a circular orbit ...
... Heisenberg also went off of Bohr’s model and elaborated more into quantum mechanics to explain the wave motion of electrons instead of a circular orbit ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.