lecture notes, page 1
... Max Born (German physicist, 1882-1970). The probability of finding a particle (the electron!) in a defined region is proportional to the square of the wavefunction. [Ψnlm(r,θ,φ)]2 = PROBABLITY DENSITY probability of finding an electron per unit volume at r, θ, φ To consider the shapes of orbitals, l ...
... Max Born (German physicist, 1882-1970). The probability of finding a particle (the electron!) in a defined region is proportional to the square of the wavefunction. [Ψnlm(r,θ,φ)]2 = PROBABLITY DENSITY probability of finding an electron per unit volume at r, θ, φ To consider the shapes of orbitals, l ...
Exam 2 with Solutions - Little Dumb Doctor .Com
... 14. In the combustion of methane, CH4, what change in hybridization (if any) occurs to the carbon atom? d. sp3 to sp 15. What type of hybrid orbital set is used by the sulfur atom in the compound SF6? e. sp3d2 16. Consider the diatomic molecules of the second period Li2, Be2, and C2. Which is (are) ...
... 14. In the combustion of methane, CH4, what change in hybridization (if any) occurs to the carbon atom? d. sp3 to sp 15. What type of hybrid orbital set is used by the sulfur atom in the compound SF6? e. sp3d2 16. Consider the diatomic molecules of the second period Li2, Be2, and C2. Which is (are) ...
Chapter 8 Study Guide
... c. Atoms of different elements differ in their physical and chemical properties. d. Atoms of different elements combine in simple, whole number ratios to form compounds e. In chemical reactions, atoms are combined, separated, or rearranged but never created, destroyed, or changed. 3. Describe how Th ...
... c. Atoms of different elements differ in their physical and chemical properties. d. Atoms of different elements combine in simple, whole number ratios to form compounds e. In chemical reactions, atoms are combined, separated, or rearranged but never created, destroyed, or changed. 3. Describe how Th ...
Quantum Number
... Atomic Orbitals A wave function with a given set of these three quantum numbers is called an atomic orbital ...
... Atomic Orbitals A wave function with a given set of these three quantum numbers is called an atomic orbital ...
Ch. 5 PPT Part 3
... – So if there are two electrons in one orbital, they spin in opposite directions ...
... – So if there are two electrons in one orbital, they spin in opposite directions ...
spectral lines
... This led to the classic model of the atom- similar to the solar system Distant electrons orbit a massive nucleus due to electrical forces of attraction. Rutherford’s model was very appealing but there were some “minor” problems that had to be solved. What held the nucleus together to be so s ...
... This led to the classic model of the atom- similar to the solar system Distant electrons orbit a massive nucleus due to electrical forces of attraction. Rutherford’s model was very appealing but there were some “minor” problems that had to be solved. What held the nucleus together to be so s ...
Quantum Numbers, Orbitals, Electron Configurations, Periodic Trends
... How many significant figures are in each of the following numbers? a) 34.02 c) 10.50 e) 1.2340 × 107 b) 3300 d) 0.00342 f) 12340000 Convert the numbers is questions 1a – 1d into scientific notation. a) c) b) d) Convert the following numbers that are in scientific notation into decimal form. a) 1.234 ...
... How many significant figures are in each of the following numbers? a) 34.02 c) 10.50 e) 1.2340 × 107 b) 3300 d) 0.00342 f) 12340000 Convert the numbers is questions 1a – 1d into scientific notation. a) c) b) d) Convert the following numbers that are in scientific notation into decimal form. a) 1.234 ...
Quantum Numbers, Orbitals, Electron Configurations, Periodic Trends
... How many significant figures are in each of the following numbers? a) 34.02 c) 10.50 e) 1.2340 × 107 b) 3300 d) 0.00342 f) 12340000 Convert the numbers is questions 1a – 1d into scientific notation. a) c) b) d) Convert the following numbers that are in scientific notation into decimal form. a) 1.234 ...
... How many significant figures are in each of the following numbers? a) 34.02 c) 10.50 e) 1.2340 × 107 b) 3300 d) 0.00342 f) 12340000 Convert the numbers is questions 1a – 1d into scientific notation. a) c) b) d) Convert the following numbers that are in scientific notation into decimal form. a) 1.234 ...
quantum mechanical model
... • Describe what the quantum mechanical model determines about the electrons in an atom. • Explain how sublevels of principal energy levels differ ...
... • Describe what the quantum mechanical model determines about the electrons in an atom. • Explain how sublevels of principal energy levels differ ...
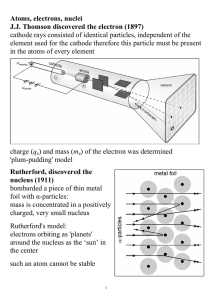
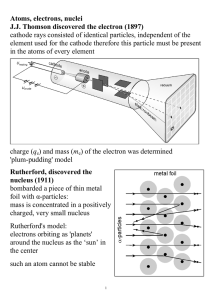
Atoms, electrons, nuclei J.J. Thomson discovered the electron (1897
... Davisson and Germer (1927) used electron beams to induce diffraction through a thin metal foil: interference interference phenomena have been shown with various other particles: duality is a general characteristic of matter Bohr's model (incorrect, but useful) electrons in an atom can only occupy ce ...
... Davisson and Germer (1927) used electron beams to induce diffraction through a thin metal foil: interference interference phenomena have been shown with various other particles: duality is a general characteristic of matter Bohr's model (incorrect, but useful) electrons in an atom can only occupy ce ...
Notes
... before going onto a different type of room. 4. When filling rooms on a floor, you must place one student in each type of room before pairing them. ...
... before going onto a different type of room. 4. When filling rooms on a floor, you must place one student in each type of room before pairing them. ...
Simple Harmonic Oscillator
... Never express yourself more clearly than you are able to think. Prediction is very difficult, especially about the future. - Niels Bohr ...
... Never express yourself more clearly than you are able to think. Prediction is very difficult, especially about the future. - Niels Bohr ...
Academic Chemistry Atomic History Study Guide 1. Identify and
... eventually lead to the production of nuclear weapons, provided insight into the internal structure and composition of the atomic nucleus. Describe their discovery. 16. _______________ ____________________ developed mathematical equations which allowed super computers to calculate the probability of ...
... eventually lead to the production of nuclear weapons, provided insight into the internal structure and composition of the atomic nucleus. Describe their discovery. 16. _______________ ____________________ developed mathematical equations which allowed super computers to calculate the probability of ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.