Lecture 1.6 PowerPoint
... • 1.6 – I can characterize an electron based on its 4 quantum numbers (n, l, ml, and ms). I can explain what each of these numbers indicate and discuss the importance of these numbers. • 1.7 – I can describe the shape, number, and energy level of the s, p, d, and f orbitals. Furthermore, I can draw ...
... • 1.6 – I can characterize an electron based on its 4 quantum numbers (n, l, ml, and ms). I can explain what each of these numbers indicate and discuss the importance of these numbers. • 1.7 – I can describe the shape, number, and energy level of the s, p, d, and f orbitals. Furthermore, I can draw ...
Electron Configurations
... – All orbitals within a sublevel have equal energy. (All 3 p sublevels are equal energy at any level.) – Energy sublevels within a principle energy level have different energies (s
... – All orbitals within a sublevel have equal energy. (All 3 p sublevels are equal energy at any level.) – Energy sublevels within a principle energy level have different energies (s
chemistry-study-guide-grade
... 1. Provide the meaning of each type of quantum number (principal, angular momentum, magnetic and electron spin). 2. Apply quantum number rules to determine allowable values for each type of quantum number. 3. Understand the basis of atomic orbitals. 4. Arrange atomic orbitals based upon energy level ...
... 1. Provide the meaning of each type of quantum number (principal, angular momentum, magnetic and electron spin). 2. Apply quantum number rules to determine allowable values for each type of quantum number. 3. Understand the basis of atomic orbitals. 4. Arrange atomic orbitals based upon energy level ...
key - gcisd
... 10. Rutherford - Gold foil experiment- expected all of the radiation to pass through and was very surprised when some of the particles were deflected led to the discovery of the NUCLEUS (centrally located positive charge) and the concept that the atom is mostly empty space. Also discovered the proto ...
... 10. Rutherford - Gold foil experiment- expected all of the radiation to pass through and was very surprised when some of the particles were deflected led to the discovery of the NUCLEUS (centrally located positive charge) and the concept that the atom is mostly empty space. Also discovered the proto ...
The Zeeman Effect
... The same expressions, but with ml and ms replaced by ML and MS, apply to the combined magnetic moments of several coupled electrons. When an atom has L≠0 and S≠0, these net magnetic moments are simply additive. Spin-orbit coupling (itself a magnetic effect) is usually large enough that the total ele ...
... The same expressions, but with ml and ms replaced by ML and MS, apply to the combined magnetic moments of several coupled electrons. When an atom has L≠0 and S≠0, these net magnetic moments are simply additive. Spin-orbit coupling (itself a magnetic effect) is usually large enough that the total ele ...
Quantum Theory
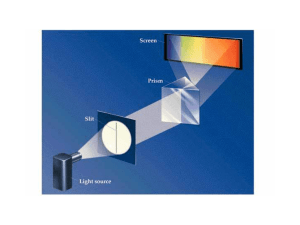
... emitted for any given element Continuous spectrum-continuous range of em light(rainbow) (Bright) Line emission spectrumOnly certain wavelengths of light are seen ...
... emitted for any given element Continuous spectrum-continuous range of em light(rainbow) (Bright) Line emission spectrumOnly certain wavelengths of light are seen ...
Electron Configuration Notes File
... Use the electron Configuration 1. draw boxes for the sublevel… 1 for s, 3 for p, 5 for d and 7 for f. 2. Fill the orbits with arrows to represent electrons. ...
... Use the electron Configuration 1. draw boxes for the sublevel… 1 for s, 3 for p, 5 for d and 7 for f. 2. Fill the orbits with arrows to represent electrons. ...
quantum numbers - Cloudfront.net
... Shape of Electron Cloud (l) Also known as sublevel or subshell Indicates the shape of the orbital within a shell Only integer values between 0 and n-1 are allowed Affects orbital energies (bigger l = higher energy) All electrons in an atom with the same value of l are said to belong to the same subs ...
... Shape of Electron Cloud (l) Also known as sublevel or subshell Indicates the shape of the orbital within a shell Only integer values between 0 and n-1 are allowed Affects orbital energies (bigger l = higher energy) All electrons in an atom with the same value of l are said to belong to the same subs ...
No Slide Title
... Dx Dp = h/4p Dx = position uncertainty Dp = momentum uncertainty (p = mv) h = Planck’s constant The uncertainty principle means that we can never simultaneously know the position (radius) and momentum (energy) of an electron, as defined in the Bohr model of the atom. ...
... Dx Dp = h/4p Dx = position uncertainty Dp = momentum uncertainty (p = mv) h = Planck’s constant The uncertainty principle means that we can never simultaneously know the position (radius) and momentum (energy) of an electron, as defined in the Bohr model of the atom. ...
Lecture 26 - Purdue Physics
... • Photons are quanta of electromagnetic radiation • Energy can be measured in electron-volts: ...
... • Photons are quanta of electromagnetic radiation • Energy can be measured in electron-volts: ...
The Wave Nature of Light
... The Nature of Energy • The wave nature of light does not explain how an object can glow when its temperature increases. • Max Planck explained it by assuming that energy comes in packets called quanta. ...
... The Nature of Energy • The wave nature of light does not explain how an object can glow when its temperature increases. • Max Planck explained it by assuming that energy comes in packets called quanta. ...
Quantum Numbers and Atomic Structure Honors
... A) total mass of all the protons and neutrons in an atom of Ti B) total mass of all the protons, neutrons, and electrons in an atom of Ti C) weighted average mass of the most abundant isotope of Ti D) weighted average mass of all the naturally occurring isotopes of Ti 5. The atomic mass of element A ...
... A) total mass of all the protons and neutrons in an atom of Ti B) total mass of all the protons, neutrons, and electrons in an atom of Ti C) weighted average mass of the most abundant isotope of Ti D) weighted average mass of all the naturally occurring isotopes of Ti 5. The atomic mass of element A ...
CH4 PT1 Arrangement of Electrons
... electrons could be particles yet they gave off waves of light. • De Broglie suggested that electrons could be considered waves confined to space around a nucleus only at specific frequencies. • Diffraction experiments proved that electron beams can interfere with each other and produce areas of low ...
... electrons could be particles yet they gave off waves of light. • De Broglie suggested that electrons could be considered waves confined to space around a nucleus only at specific frequencies. • Diffraction experiments proved that electron beams can interfere with each other and produce areas of low ...
CH 6 electrons in atoms
... thinking about the boundaries of a city. If we want to find a citizen of the city, we have a 90% probability of finding that citizen if we look in the boundaries of the city. The same is true for an electron. We believe there is a good chance or a high probability of finding the electron within the ...
... thinking about the boundaries of a city. If we want to find a citizen of the city, we have a 90% probability of finding that citizen if we look in the boundaries of the city. The same is true for an electron. We believe there is a good chance or a high probability of finding the electron within the ...
2.4. Quantum Mechanical description of hydrogen atom
... 2. i index denotes that there are several such states. The one with the lowest energy is called the ground state, the others are the excited states. About the solution: during the calculations it turns out that the states should not be labeled with a simple index i, but rather with a triplet of numb ...
... 2. i index denotes that there are several such states. The one with the lowest energy is called the ground state, the others are the excited states. About the solution: during the calculations it turns out that the states should not be labeled with a simple index i, but rather with a triplet of numb ...
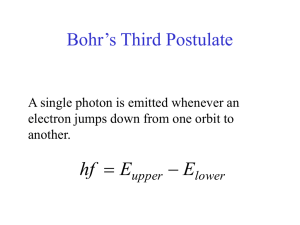
Bohr´s Third Postulate
... If we are dealing only with one photon: At any point the square of the electric field strength is a measure of the probability that a photon will be at that location. ...
... If we are dealing only with one photon: At any point the square of the electric field strength is a measure of the probability that a photon will be at that location. ...
D NAME: 1. What is the eigenvalue of Lz for Ψ if the eigenval
... Which of the following statements is/are false for a given set of QMHO wave functions corresponding to the same harmonic potential V? ...
... Which of the following statements is/are false for a given set of QMHO wave functions corresponding to the same harmonic potential V? ...
Atomic orbital
An atomic orbital is a mathematical function that describes the wave-like behavior of either one electron or a pair of electrons in an atom. This function can be used to calculate the probability of finding any electron of an atom in any specific region around the atom's nucleus. The term may also refer to the physical region or space where the electron can be calculated to be present, as defined by the particular mathematical form of the orbital.Each orbital in an atom is characterized by a unique set of values of the three quantum numbers n, ℓ, and m, which respectively correspond to the electron's energy, angular momentum, and an angular momentum vector component (the magnetic quantum number). Any orbital can be occupied by a maximum of two electrons, each with its own spin quantum number. The simple names s orbital, p orbital, d orbital and f orbital refer to orbitals with angular momentum quantum number ℓ = 0, 1, 2 and 3 respectively. These names, together with the value of n, are used to describe the electron configurations of atoms. They are derived from the description by early spectroscopists of certain series of alkali metal spectroscopic lines as sharp, principal, diffuse, and fundamental. Orbitals for ℓ > 3 continue alphabetically, omitting j (g, h, i, k, …).Atomic orbitals are the basic building blocks of the atomic orbital model (alternatively known as the electron cloud or wave mechanics model), a modern framework for visualizing the submicroscopic behavior of electrons in matter. In this model the electron cloud of a multi-electron atom may be seen as being built up (in approximation) in an electron configuration that is a product of simpler hydrogen-like atomic orbitals. The repeating periodicity of the blocks of 2, 6, 10, and 14 elements within sections of the periodic table arises naturally from the total number of electrons that occupy a complete set of s, p, d and f atomic orbitals, respectively.