ppt
... **after Dalton, there was a big hiatus in the theory of the atom. Everyone accepted Daltons theory and went on to study radiation for a while, and this is when the electron was discovered ...
... **after Dalton, there was a big hiatus in the theory of the atom. Everyone accepted Daltons theory and went on to study radiation for a while, and this is when the electron was discovered ...
Chem20u2(5.2) - Mr. Searcy Chemistry 20
... 3. Compare the Bohr and quantum mechanical models of the atom. 4. Explain the impact of de Broglie’s wave-particle duality and the Heisenberg uncertainty principle on the modern view of electrons in atoms. 5. Identify the relationships among a hydrogen atom’s energy levels, sublevels, and atomic orb ...
... 3. Compare the Bohr and quantum mechanical models of the atom. 4. Explain the impact of de Broglie’s wave-particle duality and the Heisenberg uncertainty principle on the modern view of electrons in atoms. 5. Identify the relationships among a hydrogen atom’s energy levels, sublevels, and atomic orb ...
Ch.3 lecture
... Rydberg formula (Balmer for nf = 2) : 1 / = R (1/nf2 - 1/ni2) R = Rydberg constant = 1.097 x 107 m-1 ...
... Rydberg formula (Balmer for nf = 2) : 1 / = R (1/nf2 - 1/ni2) R = Rydberg constant = 1.097 x 107 m-1 ...
Introduction to Quantum Mechanics AEP3610 Professor Scott
... the de Broglie wavelength for a moving particle, and the Born interpretion of the wave function • to ‘derive’ the Schrödinger equation(s) for said wave function for a particle in (or not in) a potential V(x) • to discuss (review?) several important potential energy cases • to explore the alternative ...
... the de Broglie wavelength for a moving particle, and the Born interpretion of the wave function • to ‘derive’ the Schrödinger equation(s) for said wave function for a particle in (or not in) a potential V(x) • to discuss (review?) several important potential energy cases • to explore the alternative ...
Ch. 5 PPT Part 2
... cosine) to the properties of the electrons – Worked for all atoms – Create electron orbitals instead of orbits – Can not pinpoint the location of the electron ...
... cosine) to the properties of the electrons – Worked for all atoms – Create electron orbitals instead of orbits – Can not pinpoint the location of the electron ...
Quantum Mechanics
... Bohr’s model has electrons in orbit around a central nucleus, but allows only certain orbits for electrons. For stable orbit, angular momentum must be a multiple of h / 2π Angular momentum of electrons is quantised ...
... Bohr’s model has electrons in orbit around a central nucleus, but allows only certain orbits for electrons. For stable orbit, angular momentum must be a multiple of h / 2π Angular momentum of electrons is quantised ...
Slide 1
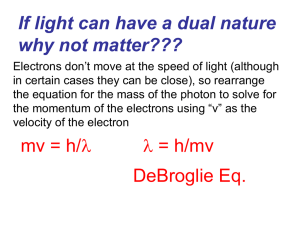
... If light can have a dual nature why not matter??? Electrons don’t move at the speed of light (although in certain cases they can be close), so rearrange the equation for the mass of the photon to solve for the momentum of the electrons using “v” as the velocity of the electron ...
... If light can have a dual nature why not matter??? Electrons don’t move at the speed of light (although in certain cases they can be close), so rearrange the equation for the mass of the photon to solve for the momentum of the electrons using “v” as the velocity of the electron ...
BORH`S DERIVATION OF BALMER
... 1.1 Rutherford’s nuclear model of the hydrogen atom Lord Earnest Rutherford [1] proposed a nuclear theory of the atom consisting of a heavy positively charged central nucleus around which a cloud of negatively charged electrons revolve in circular orbits. The hydrogen atom is the simplest, consistin ...
... 1.1 Rutherford’s nuclear model of the hydrogen atom Lord Earnest Rutherford [1] proposed a nuclear theory of the atom consisting of a heavy positively charged central nucleus around which a cloud of negatively charged electrons revolve in circular orbits. The hydrogen atom is the simplest, consistin ...
Chapter 3
... o Rutherford’s Nuclear Model o Bohr’s Planetary Model b. Explain Rutherford’s gold foil experiment and it’s significance c. Explain atomic spectra and it’s significance to Bohr’s model 2. Quantum Mechanics: a. The 4 quantum numbers and what they describe b. The difference between orbits (Bohr) and o ...
... o Rutherford’s Nuclear Model o Bohr’s Planetary Model b. Explain Rutherford’s gold foil experiment and it’s significance c. Explain atomic spectra and it’s significance to Bohr’s model 2. Quantum Mechanics: a. The 4 quantum numbers and what they describe b. The difference between orbits (Bohr) and o ...
Models of the Atom
... Why atom is stable. Accelerated electrons should emit radiation with increasing frequency as they spiral into atom. Spectra should be continuous. ...
... Why atom is stable. Accelerated electrons should emit radiation with increasing frequency as they spiral into atom. Spectra should be continuous. ...
Sec 4-1 Chapter 4 Notes
... The Rutherford model (or the planetary model) of the atom was an improvement over the previous models, but it was still incomplete. ...
... The Rutherford model (or the planetary model) of the atom was an improvement over the previous models, but it was still incomplete. ...
The Hydrogen Atom
... If we consider the vibrations of a wire loop, we find that their wavelengths always fit a whole number of times into the loop’s circumference. An electron can circle a nucleus only in orbits that contain an integral number of de Broglie wavelengths. ...
... If we consider the vibrations of a wire loop, we find that their wavelengths always fit a whole number of times into the loop’s circumference. An electron can circle a nucleus only in orbits that contain an integral number of de Broglie wavelengths. ...
In 1913 Bohr proposed his quantized shell model of the atom to
... Radiation can occur only when the electron jumps from one orbit to another. The atom will be completely stable in the state with the smallest orbit, since there is no orbit of lower energy into which the electron can jump. Bohr's starting point was to realize that classical mechanics by itself could ...
... Radiation can occur only when the electron jumps from one orbit to another. The atom will be completely stable in the state with the smallest orbit, since there is no orbit of lower energy into which the electron can jump. Bohr's starting point was to realize that classical mechanics by itself could ...
Bohr model
In atomic physics, the Rutherford–Bohr model or Bohr model, introduced by Niels Bohr in 1913, depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus—similar in structure to the solar system, but with attraction provided by electrostatic forces rather than gravity. After the cubic model (1902), the plum-pudding model (1904), the Saturnian model (1904), and the Rutherford model (1911) came the Rutherford–Bohr model or just Bohr model for short (1913). The improvement to the Rutherford model is mostly a quantum physical interpretation of it. The Bohr model has been superseded, but the quantum theory remains sound.The model's key success lay in explaining the Rydberg formula for the spectral emission lines of atomic hydrogen. While the Rydberg formula had been known experimentally, it did not gain a theoretical underpinning until the Bohr model was introduced. Not only did the Bohr model explain the reason for the structure of the Rydberg formula, it also provided a justification for its empirical results in terms of fundamental physical constants.The Bohr model is a relatively primitive model of the hydrogen atom, compared to the valence shell atom. As a theory, it can be derived as a first-order approximation of the hydrogen atom using the broader and much more accurate quantum mechanics and thus may be considered to be an obsolete scientific theory. However, because of its simplicity, and its correct results for selected systems (see below for application), the Bohr model is still commonly taught to introduce students to quantum mechanics or energy level diagrams before moving on to the more accurate, but more complex, valence shell atom. A related model was originally proposed by Arthur Erich Haas in 1910, but was rejected. The quantum theory of the period between Planck's discovery of the quantum (1900) and the advent of a full-blown quantum mechanics (1925) is often referred to as the old quantum theory.