Chapter 29 notes
... hole: vacancy left by an electron that can serve as a current carrier; acts like a positive charge. intrinsic conductivity : in a pure semiconductor, holes and electrons are always present in equal numbers, and when an electric field is applied, they move in opposite directions leading to a “natural ...
... hole: vacancy left by an electron that can serve as a current carrier; acts like a positive charge. intrinsic conductivity : in a pure semiconductor, holes and electrons are always present in equal numbers, and when an electric field is applied, they move in opposite directions leading to a “natural ...
energy levels
... • Taking part in the Manhattan Project. Bohr’s theory of hydrogen (1913): • An obsolete theory which has been replaced by quantum mechanics. • The model can still be used to develop ideas of energy and angular momentum quantization in atomic-sized systems. Assumption 1: The electron moves in circula ...
... • Taking part in the Manhattan Project. Bohr’s theory of hydrogen (1913): • An obsolete theory which has been replaced by quantum mechanics. • The model can still be used to develop ideas of energy and angular momentum quantization in atomic-sized systems. Assumption 1: The electron moves in circula ...
AP Chemistry
... Calculate the energy, in joules, required to excite a hydrogen atom by causing an electronic transition from the n = 1 to the n = 3 principal energy level. Recall that the energy levels of the H atom are given by En = 2.18 10–18 J(1/n2) ...
... Calculate the energy, in joules, required to excite a hydrogen atom by causing an electronic transition from the n = 1 to the n = 3 principal energy level. Recall that the energy levels of the H atom are given by En = 2.18 10–18 J(1/n2) ...
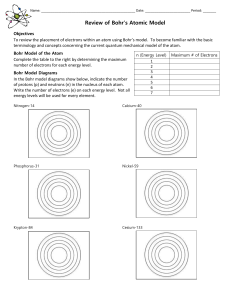
Name: ______ Date: Period: ______ Review of Bohr`s Atomic Model
... In the Bohr model diagrams show below, indicate the number of protons (p) and neutrons (n) in the nucleus of each atom. Write the number of electrons (e) on each energy level. Not all energy levels will be used for every element. ...
... In the Bohr model diagrams show below, indicate the number of protons (p) and neutrons (n) in the nucleus of each atom. Write the number of electrons (e) on each energy level. Not all energy levels will be used for every element. ...
Ch. 41 Atomic Structure
... This is consistent with our model of multielectron atoms. Bombarding an atom with a high-energy electron can knock an atomic electron out of the innermost K shell. K x rays are produced when an electron from the L shell falls into the K-shell vacancy. The energy of an electron in each shell depends ...
... This is consistent with our model of multielectron atoms. Bombarding an atom with a high-energy electron can knock an atomic electron out of the innermost K shell. K x rays are produced when an electron from the L shell falls into the K-shell vacancy. The energy of an electron in each shell depends ...
Ch 7 Lecture Notes
... Some properties of matter could not be explained by Rutherford’s model of the atom: (Dense positively charged nucleus with e- freely occupying the non-dense exterior.) 1. The presence of ______________ rather than a complete spectrum when elements were heated. ...
... Some properties of matter could not be explained by Rutherford’s model of the atom: (Dense positively charged nucleus with e- freely occupying the non-dense exterior.) 1. The presence of ______________ rather than a complete spectrum when elements were heated. ...
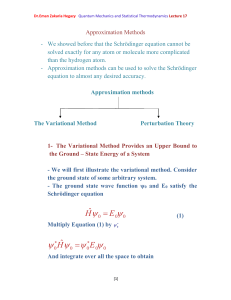
Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical
... the Ground – State Energy of a System - We will first illustrate the variational method. Consider the ground state of some arbitrary system. - The ground state wave function ψ0 and E0 satisfy the Schrödinger equation ...
... the Ground – State Energy of a System - We will first illustrate the variational method. Consider the ground state of some arbitrary system. - The ground state wave function ψ0 and E0 satisfy the Schrödinger equation ...
Bohr Model - Wikispaces
... If λ increases, f decreases. If f increases, λ decreases. Speed of the wave is always constant at 3.0 x 108 m/s. ...
... If λ increases, f decreases. If f increases, λ decreases. Speed of the wave is always constant at 3.0 x 108 m/s. ...
Electronic structure and spectroscopy
... The atomic theory allowed the development of modern chemistry, but lots of questions remained unanswered, and in particular the WHY is not being explained: • What is the binding force between atoms. It is not the charge since atoms are neutral. Why can even two atoms of the same kind (like H-H) form ...
... The atomic theory allowed the development of modern chemistry, but lots of questions remained unanswered, and in particular the WHY is not being explained: • What is the binding force between atoms. It is not the charge since atoms are neutral. Why can even two atoms of the same kind (like H-H) form ...
History of "s,p,d,f"
... The concept of spectral “terms” and the use of series names such as principal, sharp, etc., has now passed from common use, replaced by the quantitative understanding of atomic structure provided by quantum mechanics. However, the notational shorthand used by the early spectroscopists was adapted an ...
... The concept of spectral “terms” and the use of series names such as principal, sharp, etc., has now passed from common use, replaced by the quantitative understanding of atomic structure provided by quantum mechanics. However, the notational shorthand used by the early spectroscopists was adapted an ...
Atomic Structure Notes
... Section 7.8 - Electron Spin and the Pauli Principle 1. Electron spin (ms) - the fourth quantum number, developed by Goudsmit and Uhlenbeck, was necessary to account for the details of the emission spectra of atoms. The spectra indicated that the electron had a magnetic moment with two possible orien ...
... Section 7.8 - Electron Spin and the Pauli Principle 1. Electron spin (ms) - the fourth quantum number, developed by Goudsmit and Uhlenbeck, was necessary to account for the details of the emission spectra of atoms. The spectra indicated that the electron had a magnetic moment with two possible orien ...
+l - My CCSD
... – Most sources produce light that contains many wavelengths at once. – However, light emitted from pure substances may contain only a few specific wavelengths of light called a line spectrum (as opposed to a continuous spectrum). – Atomic emission spectra are inverses of atomic absorption spectra. H ...
... – Most sources produce light that contains many wavelengths at once. – However, light emitted from pure substances may contain only a few specific wavelengths of light called a line spectrum (as opposed to a continuous spectrum). – Atomic emission spectra are inverses of atomic absorption spectra. H ...
Topological Insulators
... separately, is an essential element of any future quantum computer. Scientists have succeeded in entangling many sorts of entities, typically identical atom or photon systems. But it has never been accomplished between an atomic system and a solid-state system such as a quantum dot in a semiconducto ...
... separately, is an essential element of any future quantum computer. Scientists have succeeded in entangling many sorts of entities, typically identical atom or photon systems. But it has never been accomplished between an atomic system and a solid-state system such as a quantum dot in a semiconducto ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).