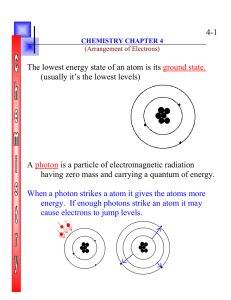
4-1 The lowest energy state of an atom is its ground state. (usually
... (usually it’s the lowest levels) ...
... (usually it’s the lowest levels) ...
Document
... de Broglie’s intriguing idea of “matter wave” (1924) Extend notation of “wave-particle duality” from light to matter For photons, P E hf h ...
... de Broglie’s intriguing idea of “matter wave” (1924) Extend notation of “wave-particle duality” from light to matter For photons, P E hf h ...
Modern Model of the Atom Student Notes and Assignment
... The ways in which electrons are arranged around the nuclei of atoms are called ELECTRON CONFIGURATIONS. The rules that govern the way the electrons fill the atomic orbitals are: 1. AUFBAU PRINCIPLE - electrons enter orbitals of the lowest energy levels first 2. PAULI EXCLUSION PRINCIPLE - an atomic ...
... The ways in which electrons are arranged around the nuclei of atoms are called ELECTRON CONFIGURATIONS. The rules that govern the way the electrons fill the atomic orbitals are: 1. AUFBAU PRINCIPLE - electrons enter orbitals of the lowest energy levels first 2. PAULI EXCLUSION PRINCIPLE - an atomic ...
Chapters 4 and 5
... Schrödinger (1926) derived an equation that treated hydrogen’s electron as a wave. Allows electron to have only certain energy but does not give path of electron. Atomic orbital – a 3-D region around the nucleus in which the electron can be found 90% of the time. ...
... Schrödinger (1926) derived an equation that treated hydrogen’s electron as a wave. Allows electron to have only certain energy but does not give path of electron. Atomic orbital – a 3-D region around the nucleus in which the electron can be found 90% of the time. ...
Review Notes - Biochemistry
... 1. Ionic Bonding: When _1_ or more electrons are _TRANSFERRED_ from one atom to another. Ion: an atom with a_CHARGE_. When an electron is gained it will be _NEGATIVE_charged and when an electron is lost it will be _POSITIVE_ charged. ...
... 1. Ionic Bonding: When _1_ or more electrons are _TRANSFERRED_ from one atom to another. Ion: an atom with a_CHARGE_. When an electron is gained it will be _NEGATIVE_charged and when an electron is lost it will be _POSITIVE_ charged. ...
Recherches sur la théorie des quanta
... positively-charged structure at its center The electrons (discovered by Thomson) must therefore exist outside of this central nucleus …. Orbiting around the nucleus as planets do the Sun. ...
... positively-charged structure at its center The electrons (discovered by Thomson) must therefore exist outside of this central nucleus …. Orbiting around the nucleus as planets do the Sun. ...
Electron Configuration and Chemical Periodicity
... – Periodic table included the 65 known elements – Mendeleev left blank spaces for the undiscovered elements and was able to predict their properties – The true basis of periodicity is the atomic number not the atomic mass (Mosley, 1913) ...
... – Periodic table included the 65 known elements – Mendeleev left blank spaces for the undiscovered elements and was able to predict their properties – The true basis of periodicity is the atomic number not the atomic mass (Mosley, 1913) ...
Problem Set II
... which couples vibrational and rotational motion. 3) The expression for the Rydberg constant on page 22 of M&S, R = mee4/8ε02ch3 = 1.097373147 x 107 m-1, is not quite correct. The hydrogen atom is a two-body problem and the correct expression is R = μe4/8ε02ch3 where μ is the reduced mass for the el ...
... which couples vibrational and rotational motion. 3) The expression for the Rydberg constant on page 22 of M&S, R = mee4/8ε02ch3 = 1.097373147 x 107 m-1, is not quite correct. The hydrogen atom is a two-body problem and the correct expression is R = μe4/8ε02ch3 where μ is the reduced mass for the el ...
Many-Electron Atoms Thornton and Rex, Ch. 8
... In principle, can now solve Sch. Eqn for any atom. In practice, -> Complicated! ...
... In principle, can now solve Sch. Eqn for any atom. In practice, -> Complicated! ...
國立嘉義大學九十七學年度轉學生招生考試試題
... 14. What is the principal quantum number of 3d z orbital? (A) 1 (B) 2 (C) 3 (D) 4 15. What is the angular momentum quantum number of 3d z orbital? (A) 1 (B) 2 (C) 3 (D) 4 16. How many quantum numbers are required to describe the electron of any systems? (A) 1 (B) 2 (C) 3 (D) 4 17. If the principal q ...
... 14. What is the principal quantum number of 3d z orbital? (A) 1 (B) 2 (C) 3 (D) 4 15. What is the angular momentum quantum number of 3d z orbital? (A) 1 (B) 2 (C) 3 (D) 4 16. How many quantum numbers are required to describe the electron of any systems? (A) 1 (B) 2 (C) 3 (D) 4 17. If the principal q ...
Spectroscopy - Birmingham City Schools
... 1924 deBroglie says: perhaps electrons are both wave and particle 1925 Erwin Schrödinger worked out equations that took wave nature of electrons into account. ...
... 1924 deBroglie says: perhaps electrons are both wave and particle 1925 Erwin Schrödinger worked out equations that took wave nature of electrons into account. ...
Atomic Theory notes
... You can’t see the actual atoms, what you can see are the electron vibrations of the atoms. The atom is mostly empty space with a nucleus. And that nucleus is 100K times smaller than the width of the atom. The model is a representation of the image of the outer electron shell. ...
... You can’t see the actual atoms, what you can see are the electron vibrations of the atoms. The atom is mostly empty space with a nucleus. And that nucleus is 100K times smaller than the width of the atom. The model is a representation of the image of the outer electron shell. ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).























