chapter 7 – cyu
... atomic number, called periodicity. When he did this, there are instances where the atomic mass of an element is heavier then the following element. This is called a reversal. 2. Modern atomic mass units are ratios of the mass of a 12 6C atom. A proton is = a.m.u. An electron is = 1/1837 a.m.u. A neu ...
... atomic number, called periodicity. When he did this, there are instances where the atomic mass of an element is heavier then the following element. This is called a reversal. 2. Modern atomic mass units are ratios of the mass of a 12 6C atom. A proton is = a.m.u. An electron is = 1/1837 a.m.u. A neu ...
Supplementary Notes on Volumetric Analysis
... (b) Which of these three compounds is/are energetically stable with respect to their constituent elements? (1M) _______________________________________________________ (c) Calculate the enthalpy change for the hypothetical reaction: 2 MgCl(s) MgCl2(s) + Mg(s) using the Hf values you calculated i ...
... (b) Which of these three compounds is/are energetically stable with respect to their constituent elements? (1M) _______________________________________________________ (c) Calculate the enthalpy change for the hypothetical reaction: 2 MgCl(s) MgCl2(s) + Mg(s) using the Hf values you calculated i ...
rutherfords model
... waves of the same frequency – The radius should steadily decrease as this radiation is given off – The electron should eventually spiral into the nucleus ...
... waves of the same frequency – The radius should steadily decrease as this radiation is given off – The electron should eventually spiral into the nucleus ...
1 - theozone
... spectrum includes light with a wavelength of 434 nanometers. This is caused by an electron moving from: a. b. c. d. ...
... spectrum includes light with a wavelength of 434 nanometers. This is caused by an electron moving from: a. b. c. d. ...
1 - Revsworld
... spectrum includes light with a wavelength of 434 nanometers. This is caused by an electron moving from: a. b. c. d. ...
... spectrum includes light with a wavelength of 434 nanometers. This is caused by an electron moving from: a. b. c. d. ...
Objective of the course Aim of the course is to introduce the basic
... Aim of the course is to introduce the basic notions of non-relativistic quantum mechanics and its interpretation. At the end of the course the students should: 1) have understood the definition of physical state and the superposition principle in quantum mechanics, the definition of physical observa ...
... Aim of the course is to introduce the basic notions of non-relativistic quantum mechanics and its interpretation. At the end of the course the students should: 1) have understood the definition of physical state and the superposition principle in quantum mechanics, the definition of physical observa ...
Review Sheet
... Atoms are ___________ because the number of _________ charged protons equals the number of ___________ charged electrons. ...
... Atoms are ___________ because the number of _________ charged protons equals the number of ___________ charged electrons. ...
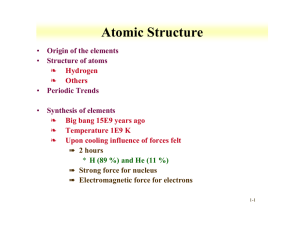
Atomic Structure Origin of the elements Structure of atoms Periodic Trends
... ❧ rapidly decay to form stable neutron rich nuclei • P process ❧ Proton capture, not as common ...
... ❧ rapidly decay to form stable neutron rich nuclei • P process ❧ Proton capture, not as common ...
Introduction to Quantum Mechanics Homework #3 (Due on April 28
... 6.1) What are the electronic configurations of Ti atom and Fe3+ ion, respectively? Hint: Consider which orbital electrons are ionized first. 2) If one performs the Stern-Gelach experiment using a Fe3+ ion beam, how many iron spots do you expect to be deposited on a glass plate after passing through ...
... 6.1) What are the electronic configurations of Ti atom and Fe3+ ion, respectively? Hint: Consider which orbital electrons are ionized first. 2) If one performs the Stern-Gelach experiment using a Fe3+ ion beam, how many iron spots do you expect to be deposited on a glass plate after passing through ...
Louie de Broglie
... Erwin Schrodinger However, the equation does not define the exact path the electron takes around the nucleus. It only estimates the probability of finding an electron in a certain position, unlike Bohr’s circular orbits. ...
... Erwin Schrodinger However, the equation does not define the exact path the electron takes around the nucleus. It only estimates the probability of finding an electron in a certain position, unlike Bohr’s circular orbits. ...
Chapter 4: Struct of Atom
... S EM radiation (e.g., line spectra) occur when electrons move from one stationary state to another. E of radiation = h*f = E1 - E2. S When moving in stationary orbits, laws of classical physics are valid, but not during transitions S Mean KE of electron-nucleus system ...
... S EM radiation (e.g., line spectra) occur when electrons move from one stationary state to another. E of radiation = h*f = E1 - E2. S When moving in stationary orbits, laws of classical physics are valid, but not during transitions S Mean KE of electron-nucleus system ...
Atoms - rcasao
... the electron must accelerate when moving in a circle and therefore radiate electromagnetic energy of frequency equal to its motion. • According to classical theory, such an atom would quickly collapse, the electron spiraling into the nucleus as it radiates away its energy. • Bohr solved the difficul ...
... the electron must accelerate when moving in a circle and therefore radiate electromagnetic energy of frequency equal to its motion. • According to classical theory, such an atom would quickly collapse, the electron spiraling into the nucleus as it radiates away its energy. • Bohr solved the difficul ...
Hydrogen atom
A hydrogen atom is an atom of the chemical element hydrogen. The electrically neutral atom contains a single positively charged proton and a single negatively charged electron bound to the nucleus by the Coulomb force. Atomic hydrogen constitutes about 75% of the elemental (baryonic) mass of the universe.In everyday life on Earth, isolated hydrogen atoms (usually called ""atomic hydrogen"" or, more precisely, ""monatomic hydrogen"") are extremely rare. Instead, hydrogen tends to combine with other atoms in compounds, or with itself to form ordinary (diatomic) hydrogen gas, H2. ""Atomic hydrogen"" and ""hydrogen atom"" in ordinary English use have overlapping, yet distinct, meanings. For example, a water molecule contains two hydrogen atoms, but does not contain atomic hydrogen (which would refer to isolated hydrogen atoms).