* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Biol 178 Lecture 4
Silencer (genetics) wikipedia , lookup
Paracrine signalling wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Signal transduction wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Genetic code wikipedia , lookup
Biosynthesis wikipedia , lookup
Ancestral sequence reconstruction wikipedia , lookup
Point mutation wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Expression vector wikipedia , lookup
Gene expression wikipedia , lookup
Magnesium transporter wikipedia , lookup
Metalloprotein wikipedia , lookup
Homology modeling wikipedia , lookup
Bimolecular fluorescence complementation wikipedia , lookup
Interactome wikipedia , lookup
Biochemistry wikipedia , lookup
Protein purification wikipedia , lookup
Western blot wikipedia , lookup
Protein–protein interaction wikipedia , lookup
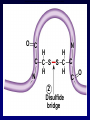
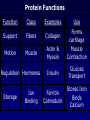
Bio 178 Lecture 4 The Chemical Building Blocks of Life Outline • • • • • Macromolecules Proteins Nucleic Acids Lipids (Mon) Carbohydrates (Mon) Reading • Chapter 3 Quiz Material • Questions on P 60 • Chapter 3 Quizzes on Text Website (www.mhhe.com/raven7) Chemistry of Carbon - Organic Molecules • Biological molecules are composed primarily of C atoms bonded to H, O, N, & S. • Hydrocarbons - Store a lot of energy! Functional Groups • The part of a molecule responsible for its chemical properties. • Organic molecules Have a C based core with attached functional groups. Functional Groups Macromolecules Types Carbohydrates, lipids, proteins, & nucleic acids. Polymer A long chain of similar molecules. Making and Breaking Macromolecules • Dehydration synthesis • Hydrolysis Proteins Composition Polymers of amino acids. Carboxyl Group Amino Acids Amino Group Functional groups can be polar, non-polar, electrically charged, aromatic, or have unique properties. Protein Synthesis Protein Structure Bonds that stabilize Protein Structure • Hydrogen bond • Disulfide bridge • Ionic bond Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Fig. 3.7b(TE Art) O C N H H C C S S C C H H O N C 2 Disulfide bridge Levels of Protein Structure • Primary • Secondary • Motifs • Tertiary • Domains • Quaternary Primary Structure • Unique aa seq of a protein • Determined by genetic information Secondary Structure of a Protein Coiling or folding of the polypeptide chain as the result of hydrogen bonds at regular intervals along the polypeptide backbone Motifs (Supersecondary structure) A distinctive, usually recurrent structural element (secondary protein structures) such as a simple protein motif consisting of two alpha helices. Examples: • turn • • barrel Tertiary Structure • The overall 3D shape of the polypeptide chain. Hydrophobic regions will be on the inside. • Due to interactions between the R groups. • Stability of tertiary structure is determined by how well non-polar R groups (will be different sizes) fit into the protein interior. Domains • Portion of a polypeptide chain that folds independently of the rest of the polypeptide chain (can be excised and still fold correctly). • Domains often have different functions within the protein (eg. DNA binding region). Quaternary Structure The spacial arrangement of the polypeptide chains (subunits) when a protein is composed of 2 or more polypeptide chains. Protein Folding Chaperone Proteins • Enable new proteins to fold correctly. • Example - heat shock proteins. • Scientific evidence suggests that the primary function of chaperones is to prevent protein aggregation (of incompletely folded proteins). Chaperone Proteins and Disease • Can chaperones prevent protein misfolding diseases? • Why do these defense mechanisms fail in patients with these diseases? Human Brain with Spongiform Encephalopathy Normal Protein Folding is Critical to Function Normal (Good) PrPC Prion (Bad) PrPSc Protein Denaturation A change in the shape of the protein. Caused by a change in temperature or polarity of the protein’s environment. Protein Functions Function Class Examples Use Enzyme catalysis Enzymes Proteases Break down protein Defense Transport Transport Mark foreign Immunoglobulins Antibodies substances Long distance transporters Hemoglobi Transport O2 n and CO2 Short distance transporters Transports protons across membranes Proton pump Protein Functions Function Support Motion Class Fibers Collagen Muscle Actin & Myosin Regulation Hormones Storage Examples Ion Binding Use Forms cartilage Muscle Contraction Insulin Glucose Transport Ferritin Calmodulin Stores Iron Binds Calcium Structural Proteins Fig. 3.4 Nucleic Acids Composition Polymers of nucleotides (5-C sugar, phosphate group, & nitrogenous base). Types • DNA (deoxyribonucleic acid) • RNA (ribonucleic acid) Functions DNA - Genetic instructions. Occurs in the nucleus. RNA - Direct protein synthesis. Made in the nucleus, travels to the cytoplasm. A Nucleotide is a monomer of a nucleic acid Nitrogenous Bases