* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download HIV-1
Genome evolution wikipedia , lookup
Human–animal hybrid wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Point mutation wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Human genetic variation wikipedia , lookup
Public health genomics wikipedia , lookup
Viral phylodynamics wikipedia , lookup
Genome (book) wikipedia , lookup
Genetic engineering wikipedia , lookup
Designer baby wikipedia , lookup
Helitron (biology) wikipedia , lookup
History of genetic engineering wikipedia , lookup
Koinophilia wikipedia , lookup
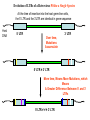
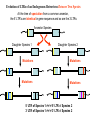
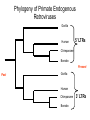
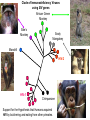
Viruses: genetic elements encased in protein • Viruses cannot reproduce independently: they are missing several of the characteristics of living organisms (no cellular organization, no growth, no independent replication). • They do cause human diseases such as influenza, polio, smallpox, and AIDS. • They cause plant diseases: e.g., more than 900 viruses are known to infect crop plants. • They cause pet diseases such as rabies, Feline Leukemia (FeLV), Feline Immunodeficiency (FIV), Canine Distemper. • Genetic engineering: viruses can be used as a tool to move genes from one organism to another. HPV, An Emerging Plant Virus • The high plains virus (HPV) was first found in 1993 infecting corn and winter wheat of the central and western USA. • By 1995, HPV was found to be widespread and cause severe symptoms or death of maize, wheat, barley, and several species of grasses. • HPV is one of a family of plant viruses that cause wheat spot mosaic, fig mosaic, thistle mosaic, rose rosette, and redbud yellow ringspot diseases • Aceria tosichella mite that transfers virus among plants. • Corn HPV resistance genes have already been mapped and may reduce mite feeding or the spread of the virus from the point of infection. Seal Plague Virus Measles Porpoise morbillivirus Dolphin morbillivirus Canine distemper • • • Seal Plague Virus (PDV) Harbor seal, Phoca vitulina 1988 Europe: population reduced to 4,000 animals by 1989. • 2002 Europe: death toll 22,00030,000 animals out of a total harbor seal population of 88,000. Viruses Replicate in Two Ways • Lytic Cycle: invades a host cell, takes over the host’s DNA replication machinery, makes more viruses. Virions then lyse host cell to escape and infect other host cells. • Lysogenic Cycle: invades host cell, incorporates into host cell DNA, replicates with host cell DNA and is transmitted to descendant host cells like a host gene. Types of Human Viral Diseases • “Hit and Run”: produce acute disease in the individual and a self-limiting epidemic in the human population, conferring immunity on infected surviving individuals. Examples: Influenza and smallpox. • “Recurrent Disease”: persists in latent form after initial infection and recurs during the life of the infected individual. Examples: Chicken pox (shingles later in life) and herpes. • “Endogenous Latency“: inserts into human germ cell lines and is transmitted from host parent to host offspring like genes for millions of generations. Examples: Human endogenous retroviruses (HERTs). Endogenous Retroviruses • Endogenous retroviruses (ERVs) have invaded the germ-line cells of every species of vertebrate and are transmitted, like genes, as part of normal host reproduction. • 8% of the human genome consists of HERVs. • Host genomes are continually evolving new regulatory mechanisms to silence the mutagenic effects on host genes associated with the replication of HERVs. This results in a reciprocal selective pressure on the HERVs to evolve to escape the host controls. HERV genes are in here, ~ 8,000 base pairs 5’ LTR 3’ LTR Long Terminal Repeats (LTRs) are each ~1,000 base pairs Evolution of LTRs of a Retrovirus Within a Single Species At the time of insertion into the host germ-line cells, the 5’ LTR and the 3’LTR are identical in gene sequence Host DNA 5’ LTR Over time, Mutations Accumulate 3’ LTR 5’ LTR ≠ 3’ LTR More time, Means More Mutations, which Means A Greater Difference Between 5’ and 3’ LTRs 5’ LTR ≠ ≠ ≠ 3’ LTR Evolution of LTRs of an Endogenous Retrovirus Between Two Species At the time of speciation from a common ancestor, the 5’ LTR’s are identical in gene sequence and so are the 3’LTRs Ancestor Species Daughter Species 1 Mutations Mutations Daughter Species 2 Mutations Mutations 5’ LTR of Species 1 ≠ ≠ ≠ 5’ LTR of Species 2 3’ LTR of Species 1 ≠ ≠ ≠ 3’ LTR of Species 2 Phylogeny of Primate Endogenous Retroviruses Gorilla Human 5’ LTRs Chimpanzee Bonobo Present Past Gorilla Human Chimpanzee Bonobo 3’ LTRs Human Immunodeficiency Virus (HIV), the cause of AIDS • HIV is a retrovirus (see Figure 26.5 in your text). • HIV is called a lenti virus, owing to the long time it takes to go from initial infection to disease. • It is transmitted from infected to uninfected persons by blood or semen. • There are two genetically different kinds of HIV circulating in the human population, HIV-1 and HIV-2 • Hypothesis: Humans acquired HIV by butchering and eating from other primates. Testing the Hypothesis that Humans acquired HIV from other primates 1. Obtain SIV gene sequences from a variety of primate species. Simian Immunodeficiency Virus = SIV 2. Compare SIV gene sequences to HIV-1 and HIV-2 gene sequences. 3. The most similar DNA sequences share a more recent common ancestor. 4. The most recent common SIV ancestor is the source of HIV: HIV-1 Has a different origin than HIV-2. Clade of Immunodeficiency Viruses using SIV genes African Green Monkey Sike’s Monkey Sooty Mangabey Mandrill HIV-2 HIV-1 Chimpanzee Support for the Hypothesis that Humans acquired HIV by butchering and eating from other primates. Refinement of the Hypothesis of the Origin of HIV-1 • • • Which population of Chimpanzees did HIV-1 come from? There are two chimpanzee subspecies in Africa: (1) Pan troglodytes troglodytes in central Africa; and, (2) P. t. schweinfurthii in eastern Africa. SIVcpz-1 is genetically very divergent from SIVcpz-2, consistent with the subspecies designation and the geographic distance between the subspecies. Genetic and Bio-geographical Evidence for Sub-Specific origin of HIV-1 • All HIV-1 strains are closely related to SIVcpz-1. = Genetic Evidence. • The natural range of P. t. troglodytes coincides uniquely with areas where the Human population of Africa harbors HIV-1. = Geographic Evidence. Conclusion: These two patterns indicate that P. t. troglodytes is the primary zoonotic reservoir for HIV-1. • Additional genetic evidence (not shown) suggests that there have been at least three independent introductions of SIVcpz-1 into the human population! Further testing of the Hypothesis • Genetic evidence supports the Hypothesis that HIV is a zoonotic infection, that is, our species has acquired HIV at least twice from another species. Once from the Chimpanzee and once from the Sooty Mangabey. • From the view-point of the virus, infecting our species is a host range expansion. • Additional Hypothesis: If Humans acquired HIV by eating ‘bush meat,’ then we should also carry other blood-borne viruses from these same primates. Simian foamy viruses (SFVs) • Simian foamy viruses (SFVs) are ubiquitous, non-pathogenic, non-endogenous retroviruses that infect all primate species. • Vertically transmitted from mother to offspring, contagiously but not in the germline. • They have co-speciated with Old World Primates for over 30 million years: Every Old World Primate Has its OWN UNIQUE SFV. Genetic Evidence of Mother-Offspring Contagious Transmission of SFV TREE or Clade from Primate Maternally Transmitted Mitochondrial DNA TREE or Clade from SFV Gene Sequence No Human FV • All foamy virus infections identified in humans are of zoonotic origin and all have occurred in persons exposed to non-human primates, like zoo keepers. • Although humans share a common evolution with non-human primates and are susceptible to SFV infection, humans are not endemically infected with a species-specific FV like other primates. • Hypothesis: HFV became extinct in humans because of reduced transmissibility from mother to offspring. • Hypothesis: HFV exists endemically in some human populations but they have not yet been tested.