* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Kathryn Davis
Pharmacokinetics wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmacognosy wikipedia , lookup
Drug interaction wikipedia , lookup
Drug design wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Neuropharmacology wikipedia , lookup
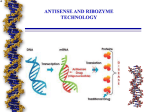
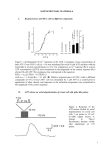
Kathryn Davis Project summary for Summer Honors Research 2003 Malfunctioning genes cause many diseases including cancer. Genes are composed of DNA whose language is made of specific sequences (ex. CTGAGTC). There are four letters used in the DNA language, C, G, A, & T. The sequences are then transcribed into mRNA and translated into proteins. The proteins carry out specific functions in the cell. In English the word CAT has a certain meaning, but if the letters are mixed up, such as ACT, then the word takes on a new meaning. In the same way if a DNA sequence is out of order then the correct protein will not be made, the correct function is not carried out, and disease is caused. A new type of therapy called antisense gene therapy has been developed to combat genetic disease. A drug (called an oligonucleotide) is bound to the cell’s malfunctioning gene and stops it from making the erroneous protein. This therapy is good for the patient, because it is not poisoning the cells like most drugs today; rather it is helping the cell so it can then heal itself. A drug that is on the market today that uses the antisense mechanism is Vitravene®, which treats cytomegaloviral retinitis, an eye disease common among AIDS patients. Oligonucleotides, short strands of DNA or RNA, can be synthesized chemically in the lab. Oligonucleotides have been tested as antisense drugs, and have been shown to regulate the expression of disease. Different applications of these antisense oligonucleotides include therapy for hypertension, cardiovascular disease, autoimmune disease, parasitic infections (such as HIV), and cancer. The mechanisms of antisense drugs have been studied, but there is still a question as to which mechanism these drugs use (4). Mechanisms include the oligonucleotide binding to the mRNA and blocking translation (a true antisense function); the oligonucleotide binding to the DNA halting transcription; RNase H nuclease induction: which destroys mRNA; and the oligonucleotide binding to the active protein and blocking its action. Modifications to the DNA oligonucleotides help these potential drugs drug to last longer in the cell. Oligonucleotides that are modified are important because the cell is less likely to recognize them as something foreign and destroy them. For these reasons it is important to make oligonucleotides more cost effective so that we can better understand their mechanisms. There is much more research to be done in this field. Modifications such as a sulfur group in place of one of the non-bridging oxygen atoms in the phosphodiester backbone of the DNA (mono-thio) are currently being used in the antisense drug effort to aid in their effectiveness (Vitravene® is one example). Many other modifications are also being researched, including the dithio phosphate modification in which both of the non-bridging oxygens in the DNA backbone are replaced with sulfurs. This modification, however, has a very costly and difficult precursor synthesis and loss during purification is high. The dithio-modified oligonulceotides could make the drug even more effective, but dithio-modified oligonucleotides have not been studied as much due to the cost and difficulty of their purification. 5' 5' O P S O- O O O 3' Base Monothiophosphate O Phosphodiester O Base O Base O P O- O O O 3' Base In order for an oligonucleotide to be used as an antisense drug it must be very pure. However due to other interactions during purification, methods typically result in only 70% of expected product yield. Failure sequences that would interfere with the drug’s effectiveness are removed during HPLC. This is yet another the reason why the oligonucleotides are so expensive to study. Pharmaceutical companies put pressure on researchers to create more efficient methods of developing and synthesizing new drugs. Few companies have taken any measures to increase the efficiency and lower the cost at the purification level (1). My honors summer research project was focused on increasing the efficiency of the purification step in the production of monothiophosphate DNA in comparison to nonmodified oligonucleotides of the same and different sequences. My hypothesis was that as the number of sulfur modifications increased the percent yield would be less, due to the sulfur binding to the metal tubing in the HPLC. Also I hypothesized that the oligonucleotide with a more complex sequence would have a lower product yield. During my research I synthesized oligonucleotides of four different sequences, using the Gene Assembler Special/4 Primers from Pharmacia and post synthetic procedures (2). The oligonucleotides differed in sequence, and amount of mono-thio modification. 5’-TTTTTT-3’ 5’-CTCTCTCTCT-3’ 5’-CTCTC*TCTCT-3’ 5’-C*T*C*T*C*T*C*T*C*T-3’ * = monothio modification After synthesizing the oligonucleotides, I determined the amount of DNA in the sample using the UV-vis spectrophotometer. The oligonucleotides were purified using reversed phase high-pressure liquid chromatography (HPLC). After HPLC the purified sample was quantified again using the UV-Vis. Percent yield was calculated. Each sequence was tested three times. Based on my research I found that loss was inconsistent due to the sequence differences as well as the amount of modification (which indicates that the method needs to be studied further). There was a decrease in loss from zero sulfur modification to one sulfur modification (opposite of hypothesis). However there was an increase in loss from one sulfur modification to nine sulfur modifications (consistent with hypothesis). Sequence Trial # 5'-TTTTTTTTTT-3' 5'-TTTTTTTTTT-3' 5'-TTTTTTTTTT-3' 5'-CTCTCTCTCT-3' 5'-CTCTCTCTCT-3' 5'-CTCTCTCTCT-3' 5'-CTCTC*TCTCT-3' 5'-CTCTC*TCTCT-3' 5'-CTCTC*TCTCT-3' 5'-C*T*C*T*C*T*C*T*C*T-3' 5'-C*T*C*T*C*T*C*T*C*T-3' 5'-C*T*C*T*C*T*C*T*C*T-3' Three trial Final % yield % Lost average 1 33.80% 66.20% 2 43.00% 57.00% 50.03% 3 73.10% 26.90% 1 46.10% 53.90% 2 10.00% 90.00% 63.60% 3 53.10% 46.90% 1 54.20% 45.80% 2 73.20% 26.80% 33.00% 3 73.60% 26.40% 1 64.20% 35.80% 2 61.00% 39.00% 38.90% 3 58.10% 41.90% Further research will be needed to reconcile the inconsistencies and variance in the results. Additional research could also include: a comparative study of the Waters 510 pumps system (all stainless steel system used in this study) to the Waters 626 system (a polymeric pump system); synthesizing a step-wise series of the modified oligonucleotides; doing a comparison of different methods of purification; testing out new synthetic methods that are being developed; and synthesizing oligonucleotides with different types of modifications. Bibliography 1. Bennett, CF. “Efficiency of antisense oligonucleotide drug discovery.” Antisense Nucleic Acid Drug Development Jun. 2002; 12(3): 215-224. 2. Davis, Kathryn. HSRP 2003 paper 3. Gene Assembler Special/4 Primers User Manual: Edition AC 4. Oligonucleotides as Therapeutic Agents- Symposium No. 209. New York: John Wiley & Sons, Inc., 2000-2003.