* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Slide 28
Particle in a box wikipedia , lookup
X-ray fluorescence wikipedia , lookup
EPR paradox wikipedia , lookup
Ferromagnetism wikipedia , lookup
Wave–particle duality wikipedia , lookup
Chemical bond wikipedia , lookup
Molecular orbital wikipedia , lookup
Tight binding wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
Atomic theory wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Hydrogen atom wikipedia , lookup
Electron-beam lithography wikipedia , lookup
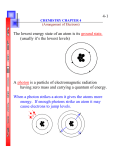
Presentation Details: Slides: 28 Duration: 00:05:58 Filename: C:\Users\jpage\Documents\NCVPS Learning Objects\Hon Chem Electron Configuration Navigation to PPT W\Electron configuration mod 2 Lesson 3 Navagational ppt. and audio\Module 2 Lesson 3 Electron configuration.ppt Presenter Details: Electron Configuration and Basic Quantum Model NC Essential Standard 1.3.2 Quantum mechanics and it relation to electron configuration Published by Articulate® Presenter www.articulate.com Slide 1 Notes: Electron Configuration and Basic Quantum Model This lesson is on how the electrons are arranged and basic quantum mechanics. Duration: 00:00:05 Advance mode: Auto Electron Configuration and Basic Quantum Model NC Essential Standard 1.3.2 Quantum mechanics and it relation to electron configuration Slide 2 Notes: Quantum Model Quantum Model Duration: 00:00:33 Advance mode: Auto • • • The quantum model describes the probability of locating an electron at any place. Heisenberg Uncertainty Principle – it is impossible to know both the velocity (momentum) and the position of an electron at the same time. – The impact of a photon of light alters the motion of the electron in unpredictable ways, so measuring position changes velocity Each electron is assigned four quantum numbers that describe it. No two electrons of an atom can have the same four quantum numbers. Slide 3 Notes: Principle Energy Level, n Duration: 00:00:16 Advance mode: Auto Principle Energy Level, n • • • • • • • • Published by Articulate® Presenter The quantum model describes the probability of locating an electron at any place. The Heisenberg Uncertainty Principle – states it is impossible to know both the velocity (momentum) and the position of an electron at the same time The impact of a photon of light alters the motion of the electron in unpredictable ways, so measuring position changes velocity. Each electron is assigned four quantum numbers that describe its location within the atom. No two electrons of an atom can have the same four quantum numbers. Indicates main energy level occupied by eAlways a whole number (1, 2, …) To calculated the number of electrons that a given energy level can have. Simpley use the formula 2n2 1st level can hold 2(1)2 = 2 e2nd level can hold 2(2)2 = 8 e3rd level can hold 2(3)2 = 18 e4th level can hold 2(4)2 = 32 eThe period indicates the # of principle energy levels The principle quantum number is represented by the letter “n”. The n represents the energy level being looked at as well as the number of sublevels the energy level can have. By using 2 the formula 2n the number of electrons that an energy level can hold can be determined. www.articulate.com Slide 4 Notes: Sublevels, l Sublevels, l Duration: 00:00:23 Advance mode: Auto • • Main energy levels are then divided into sublevels Indicates the shape or type of orbital – s, p, d, f – s sublevel is spherical and holds 2 e– p sublevel is shaped like a dumbbell and holds 6 e– d holds 10 e– f holds 14 e- Slide 5 Notes: Orbitals, m Duration: 00:00:14 Advance mode: Auto Orbitals, m • • Represents the orbital within the sublevel where the electron is located Each orbital holds a pair of electrons therefore: – 1 s orbital – 3 p orbitals – 5 d orbitals – 7 f orbitals Slide 6 Published by Articulate® Presenter The orbitals are the actual place electrons are located. Each sublevel has a maximum number of orbitals. The s has one orbit, the p has three orbits, the d has five orbits and the f has seven orbits. Notes: Spin, s Duration: 00:00:19 Advance mode: Auto There are four shapes of sublevels which are represented by the letter (l). The sublevels are filled from lowest to highest energy. The lowest energy sublevel is s which will hold 2 electrons. The next is p which will hold 6 electrons. The next sublevel is d which will hold 10 electrons and finally the fourth sublevel is f and it will hold 14 electrons. Spin, s • Each orbital holds 2 electrons that will always spin in opposite directions the electrons are represented by +1/2 and -1/2. Note arrows are used in the orbital notation for example ??. The last quantum number is called the spin quantum number represented by the letter “s” it has two orientations. The number values are positive one half and negative one half. The up arrow indicates an electron is spinning clockwise and the down arrow indicates an electron spinning in the counter clockwise manner. www.articulate.com Slide 7 s Orbital • The s orbital is spherically shaped. There is one s orbital for each value n = 1,2,3,…, of the principle number. Duration: 00:00:07 Advance mode: Auto Slide 8 These are the three orientations for the orbits in the p sublevel. Notice each orbit is in a different orientation in space. Duration: 00:00:09 Advance mode: Auto Slide 9 Notes: d Orbital Published by Articulate® Presenter The s orbital is spherically shaped. There is one s orbital for each value of n. Notes: p Orbital Duration: 00:00:08 Advance mode: Auto Notes: • For each of the values n = 3, 4, 5,…, there are five d orbitals. Four of the five have similar shapes, but differ in orientation. These are the five orientations for the orbits in the d sublevel. Notice each orbit is in a different orientation in space. www.articulate.com Slide 10 Electron Configurations Duration: 00:00:16 Advance mode: Auto Electron Configurations • • Slide 11 Electron Configurations Duration: 00:00:33 Advance mode: Auto Slide 12 Explanation of configuration Duration: 00:00:12 Advance mode: Auto Published by Articulate® Presenter Shows the electron arrangement in an atom, always represents the lowest possible energies Aufbau Principle (German for “building up”) – electrons fill orbitals that have the lowest energies first Notes: Electron configurations are a way to show the arrangement of an atoms electrons from the lowest energy level to the highest energy level. The Augbau Principle is a governing principle that say that electrons will fill the lowest energy level that can receive it first. Notes: The relative energies of orbitals are represented here in two ways. The yellow brick road is the typical representation of the energy arrangement. The way to use the yellow brick road is to connect the arrows from right to left. The order for electrons is as follows. 1s,2s,2p,3s, 3p, 4s, 3d, and so on. The green image, lines them up in a simple flow diagram for you to follow. Note as the atom get larger, they start to overlap. Notes: The large number represents the energy level, the small letter represents the sublevel and its shape and the superscript number represents the number of electrons in the orbit. www.articulate.com Slide 13 Practice Write electron configurations Duration: 00:00:07 Advance mode: Auto Practice Write electron configurations – Pb: • 1s22s22p63s23p6 Note All the superscripts should add up to the number of electrons Slide 14 Here are some examples of how electron configurations are done. We just need to account for all the electrons and their locations. Since they fill up the lower levels first, only the last shell (Valence electrons) varies from the previous element’s configuration Duration: 00:00:17 Advance mode: Auto Slide 15 Notes: Practice Published by Articulate® Presenter The first representation of electron configuration shows the location of each of the atoms electrons. Notes: Electron Configurations Duration: 00:00:07 Advance mode: By user Notes: Practice • • • • • • • • • Write the electron configuration for C N O F Ne Na Mg Al Here is some practice for you to try. Once you have finished go to the next slide and check your answers. www.articulate.com Slide 16 Notes: Practice Duration: 00:00:04 Advance mode: By user Practice • • • • • • • • • Write the electron configuration for C: 1s2 2s2 2p2 N: 1s2 2s2 2p3 O: 1s2 2s2 2p4 F: 1s2 2s2 2p5 Ne: 1s2 2s2 2p6 Na: 1s2 2s2 2p6 3s1 Mg: 1s2 2s2 2p6 3s2 Al: 1s2 2s2 2p6 3s2 3p1 Slide 17 Refer to this Periodic Table when writing electron configurations Duration: 00:00:37 Advance mode: Auto Here are the answers from the previous slide. Notes: Refer to this Periodic Table when writing electron configurations The numbers in front of the “s” and “p” are the same as the row # P1 p2 p3 p4 p5 p6 Refer to this periodic table. The number in front of the s and p sublevel corresponds to the row. The number in front of the d sublevel is 1 behind the row number. The number in front of the f sublevel is 2 behind the row number. The exponents represent the number of electrons in 1 that particular sublevel, so you can have an s or 2 1 6 1 10 1 14 s , a p through p , d – d , and f – f These regions are called the s, p, d, f, blocks. Slide 18 Shorthand Notation Duration: 00:00:11 Advance mode: Auto Published by Articulate® Presenter Notes: The second representation is called a shorthand notation which substitutes in a noble gas symbol to represent previously filled energy levels. www.articulate.com Slide 19 Notes: Examples Here are some examples of shorthand notation. Duration: 00:00:04 Advance mode: Auto Slide 20 Notes: Practice Shorthand Notation Practice Duration: 00:00:06 Advance mode: By user Practice using shorthand notation and then check you answers with the next slide. B Ar K Ca H Mg Slide 21 Practice Answers Duration: 00:00:07 Advance mode: By user Published by Articulate® Presenter Notes: Here are the answers to the shorthand notation practice. Check over your work and redo if necessary. www.articulate.com Slide 22 Notes: Orbital notation Orbital notation uses lines to represent orbits. Below the line is number of the energy level and the letter that represents the sublevel Lastly the arrows represent the spin of each electron. Duration: 00:00:13 Advance mode: Auto Slide 23 Notes: Orbital Notation Duration: 00:00:10 Advance mode: Auto Orbital Notation • Orbital notation uses lines to represent orbits and arrows to represent the spin of each electron. • oxygen has a total of 8 e- The next governing law is Hund’s rule. This rule states that multiple orbits with the same energy will each receive one electron before any of them receive a second. The e- configuration for O: 1s2 2s2 2p4 Hund’s Rule: e- spread out within equivalent orbitals ?? ?? ?? ? ? 1s 2s 3s 3s 3p Slide 24 Sample Orbital Notation Examples Duration: 00:00:13 Advance mode: Auto He Be Notes: Here are some examples of orbital notation. Notice that Fluorine is in the excited state. We can tell by the missing arrow in 2s has moved to make the sixth electron in 2p. N F Published by Articulate® Presenter www.articulate.com Slide 25 Practice Orbital Notation Practice Practice Duration: 00:00:06 Advance mode: By user Element Notes: Practice this orbital notation then check your answers with the next slide. Orbital Notation Li C Al O Slide 26 Notes: Practice Answers Check your answers with these and if necessary redo. Duration: 00:00:05 Advance mode: By user Slide 27 Mixed practice Mixed Practice Duration: 00:00:12 Advance mode: By user Symbol Group# Valence e[Ar]4s2 [Ne]3s23p3 Notes: Period S,p,d,f block Highest energy level The electron configuration can help you determine information about the atoms identity. Use you periodic table to fill in this chart. The check your answers with the next slide. [He]2s22p5 1s22s2 1s22s22p3 1s22s22p63s23p1 Published by Articulate® Presenter www.articulate.com Slide 28 Notes: Practice Answers Duration: 00:00:05 Advance mode: By user Published by Articulate® Presenter [Ar]4s2 Symbol Group# Valence Period S,p,d,f eblock Highest energy level Ca 2 2 4 s 4 [Ne]3s23p3 P 5A /15 5 3 p 3 [He]2s22p5 O 6A /16 7 2 p 4 1s22s2 Be 2 2 2 s 2 1s22s22p3 N 5A /15 5 2 p 2 1s22s22p63s23p1 Al 3A /13 3 3 p 3 Check your answers to see how you have done. If necessary redo. www.articulate.com