* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Classification of
Stoichiometry wikipedia , lookup
Photopolymer wikipedia , lookup
Registration, Evaluation, Authorisation and Restriction of Chemicals wikipedia , lookup
Chemical reaction wikipedia , lookup
Water pollution wikipedia , lookup
Lewis acid catalysis wikipedia , lookup
Water splitting wikipedia , lookup
Biochemistry wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Abundance of the chemical elements wikipedia , lookup
Electrolysis of water wikipedia , lookup
Liquid–liquid extraction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Crystallization wikipedia , lookup
Gas chromatography wikipedia , lookup
Freshwater environmental quality parameters wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Chemical element wikipedia , lookup
Acid–base reaction wikipedia , lookup
Vapor–liquid equilibrium wikipedia , lookup
Periodic table wikipedia , lookup
History of chemistry wikipedia , lookup
Atomic theory wikipedia , lookup
Extended periodic table wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
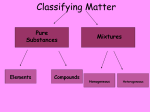
Chemistry Unit Review Name _______________________ 1. Write the term next to its definition. Choose from these terms: atom chemistry compound element heterogeneous homogeneous mixture period a) b) c) d) e) f) g) h) i) group _______period_______________________ - row on the periodic table ________group______________________ - column on the periodic table _________atom_____________________ - smallest particle of matter __________element____________________ - simplest form of matter _____________compound__________ - 2 or more elements chemically combined __________mixture_______________ - 2 or more substances physically combined _________heterogeneous______________ - mixture with individual parts visible ______homogeneous_______________ - mixture that looks the same throughout ____chemistry________________ - the study of matter 2. What is Mendeleev known for? _______designing the Periodic Table________ 3. How is the periodic table organized? from left to right, increasing by atomic number (Other answers: grouped into columns by similar characteristics; metals from left to center, non-metals on the right, metalloids on the zig-zag staircase) 4. Fill out the missing information in the table shown below: Atomic # Element Symbol Group Period 79 gold Au 11 6 80 mercury Hg 12 6 8 oxygen O 16 2 5. Use the table to answer the questions below. Substance water wood ice aluminum Top Bottom Density (g/mL) 1.00 0.40 0.92 2.70 Floats in water __wood, ice_____________________ Sinks in water _____aluminum___________________ Most dense __________aluminum_________________ Least dense ________wood_____________________ The 4 substances from the table are mixed together. Fill in the boxes to show how they would layer from top to bottom. 6. Examine the pictures of mixtures shown below. Place a checkmark in the correct space after deciding if each mixture is heterogeneous or homogeneous. Pepperoni Pizza _X_heterogeneous ___homogeneous Gravel (rocks) _X__heterogeneous ___homogeneous Stainless Steel ___heterogeneous _X__homogeneous Oatmeal Raisin Cookie _X_heterogeneous ___homogeneous Vanilla Ice Cream ___heterogeneous __X_homogeneous Cherry KoolAid Cookies and Cream Ice Cream _X__heterogeneous ___homogeneous Salt Water ___heterogeneous _X__homogeneous ___heterogeneous _X__homogeneous 7. Identify other family members for each of these elements. a) He (helium) - Group _18___ What is special about this group: ____not reactive_____________ 2 other elements in this same group: Ne, Ar, Kr, Xe, Rn, Uuo b) Li (Lithium) - Group __1__ What is special about this group: ____most reactive____ 2 other elements in this same group: _H, Na, K, Rb, Cs, Fr________ 8. Examine the pictures of substances shown below. Label each substance as an element, compound or mixture. a)____element______ b)__compound_______ c)___mixture____ 9. A compound has ________different_______________ than those of the elements used to make it. (same or different) properties 10. Write the term next to its definition. Choose from the terms in the box. acid element homogeneous solid atom base compound heterogeneous liquid molecule neutral mixture soluble*** gas indicator oxidation precipitate insoluble*** states of matter density a) _________atom____________________ - smallest particle of matter b) __________element___________________ - simplest form of matter c) _________compound_________________ - 2 or more elements whose atoms have chemically combined d) ____________mixture_____________ - 2 or more substances physically combined e) ______heterogeneous_________________ - mixture with individual parts visible f) _______states of matter_______________________ - solid, liquid, gas g) ___________solid___________________ - definite volume and shape h) ____________liquid__________________ - definite volume, changeable shape i) ____________gas__________________ - changeable volume and shape j) ______________homogeneous___ - mixture that appears the same throughout k) __________soluble____________________ - able to dissolve*** l) __________insoluble____________________ - NOT able to dissolve*** m) _______acid_______________________ - pH less than 7, lemon juice is an example n) __________base________________ - pH greater than 7, baking soda is an example o) __________neutral____________________ - pH = 7, pure water is an example p) _______oxidation_______________________ - chemical reaction with oxygen q) ______precipitate______________ - new solid created from two liquids 11. What is the change in state for each of these phase changes? a) melting – going from _____solid______________ to _________liquid____________ b) evaporation - going from _____liquid_____________ to ______gas______________ c) condensation - going from ______gas_____________ to ________liquid_________ d) freezing - going from ________liquid________________ to ______solid__________________ 12. Name an example of a solution. _______salt______________ + _______water______________ = ______saltwater_______ Solute Solvent Solution 13. Answer these questions about acids and bases. a) Name two acids. _____lemon juice_____________ & ________vinegar____________ b) Name two bases. ______ammonia_______________ & _________baking soda________ c) What is the pH range of acids? ____1____ - _____6____ bases? ___8___ - ____14___ d) What is the pH of a neutral solution? ___7______ 14. Examine the following table, and then answer the questions below. Substance Type of Matter Boiling Point (°C) Melting Point (°C) aluminum Element 2,519 660 mercury Element 357 -39 Sodium chloride Compound 1,413 801 water Compound 100 0 a) Write the element with the highest boiling point. ____aluminum______________ b) Write the compound with the lowest melting point. _____water____________ c) Which phase change is happening at the boiling point? __evaporation (L -> G)_ 15. Identify the 5 ways you can know that a chemical reaction has taken place. a) ____production of an odor_____ b) ______change in temperature____ c) _______change in color__________ d) _formation of bubbles (gas release)__ e) ____formation of a solid (precipitate)_ 16. Match these experiments to the evidence that a chemical reaction took place. Put the letters (a-e) from question #15 in the appropriate blank. Chalk Lab _____e______________ Citric acid, Baking Soda, & Vinegar ______b,d______________ Hot Milk & Vinegar _______e_____________ Cabbage Juice, Calcium Chloride, & Baking Soda _______b,c,d_____________ 17. Match the Inquiry vocabulary with the definition. Independent variable __c___ a. the variable the scientist measures (the effect) Dependent variable __a___ b. the variable the scientist keeps the same for all tests Constant/control variable _b____ c. the variable the scientist changes (the cause)