* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Ch. 19: Electric charges, Forces, and Fields (Dr. Andrei Galiautdinov, UGA)
Casimir effect wikipedia , lookup
Electromagnet wikipedia , lookup
Work (physics) wikipedia , lookup
Renormalization wikipedia , lookup
Time in physics wikipedia , lookup
Magnetic monopole wikipedia , lookup
Introduction to gauge theory wikipedia , lookup
History of electromagnetic theory wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
History of quantum field theory wikipedia , lookup
Standard Model wikipedia , lookup
History of subatomic physics wikipedia , lookup
Elementary particle wikipedia , lookup
Maxwell's equations wikipedia , lookup
Mathematical formulation of the Standard Model wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Speed of gravity wikipedia , lookup
Fundamental interaction wikipedia , lookup
Electromagnetism wikipedia , lookup
Field (physics) wikipedia , lookup
Atomic theory wikipedia , lookup
Lorentz force wikipedia , lookup
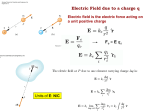
Ch. 19: Electric charges, Forces, and Fields (Dr. Andrei Galiautdinov, UGA) 2014FALL - PHYS1112 Paper & comb demo… 1 The most basic electrical phenomenon… static electricity • The silk handkerchief exhibits a static cling to a cotton shirt in the dryer. • The door knob provides a shock after scuffing your feet on the carpet. • Sparks fly when you pull the wool sweater off. • A lightning strikes during a storm. 2 Girl with a balloon… 3 4 5 6 Hair…up in the air 7 What do you think happened here? 8 What do you think happened here? 9 An important person in the history of the human kind… 10 11 …was the balloon rubbed on Donald’s hair? 12 …was the balloon rubbed on Donald’s hair? unlikely… 13 …my guess is, these are polarization charges 14 15 16 17 18 19 20 Two balloons… 21 22 Were they charged by rubbing against each other? 23 Both balloons are NEGATIVELY charged (must have been charged separately) 24 Kids on playground slides… 25 26 Plastic playground slides create enough static to fry hearing implants! Most kids have no problem with the static electricity created from sliding down plastic slides. For children with cochlear implants it's more complicated, though. The static can shut down the cochlear implant instantly. Cochlear implants were first introduced in the 1980s and have always had problems with static electricity. In the beginning, they could be shut down by simply putting on a sweater. Now they are more stable, but they can still shut down with static from slides and balloons. When the cochlear implant is shut down it costs $1,000 to be restored. It can also take days to get it done, leaving the child deaf for days. A company in Missouri that is developing anti-static coating for the Navy is seeing if their product will work on slides. They believe that they could produce it for slides at an affordable price. Metal slides aren’t terribly helpful even though they don’t produce static, because they get too hot to slide down in warm weather. 27 Lightning we’ll discuss this later, after introducing the notion of the electric field and the phenomenon of air breakdown 28 A bit of history… ancient Greeks: William Gilbert: 1. 2. Electrification is not limited to amber; it’s a general phenomenon Amber (by wool) + feather Magnetite (Fe2O3) + iron - 700 0 Charles Dufay (King of France’s gardener): 1733 1600 Hans Oersted: Michael Faraday: Inverse-square force law for electricity Connection b/w electricity and magnetism (compass needle is deflected by current) 1. 2. Heinrich Hertz: Produced EM waves in the lab 1887 Concept of E & M fields EM Induction (changing magnetic field produces current in a circuit) 1831 1820 Alexander Popov Joseph Thomson: Guglielmo Marconi: Discovery of the Radio electron 1896 1. + and – electricity 2. Likes repel, opposites attract Electrically charged objects can also repel each other Charles Coulomb: 1785 Benjamin Franklin: 1897 1750 James Clerk Maxwell: 1. Laws of E&M in modern form 2. Existence of EM waves 3. Light is an EM wave 1865 to 1873 P. N. Lebedev: E. Rutherford: Niels Bohr: Light pressure “planetary” model of atom “(semi-) quantum” model of atom 1900 1911 1913 Charles-Augustin de Coulomb (14 June 1736 – 23 August 1806) was a French physicist. He is best known for developing Coulomb's law (the inverse-square law of electrostatics). The SI unit of electric charge, the coulomb, was named after him. 30 31 Unit of charge Name: coulomb [C] Definition (different from the technical SI definition): 1 [C] = charge of (6.242 x 1018) protons = (6.242 x 1018 ) qp, where qp is the basic atomic unit of charge. Thus, qp = 1.602 x 10-19 [C] Note: electron charge is qe = (- qp) = - 1.602 x 10-19 [C] 32 Coulomb’s Law Coulomb’s law gives the force between two point charges: The force is along the line connecting the charges, and is attractive if the charges are opposite, and repulsive if the charges are like. Coulomb’s Law The forces on the two charges are action-reaction forces. Superposition principle If there are multiple point charges, the forces add by superposition. 1 [C] is a huge amount of charge. Here’s an example: 36 1 [C] is a huge amount of charge. Here’s an example: 37 38 39 40 2 41 42 43 The Field Concept (took 2,500 years to arrive at) A bit of history… ancient Greeks: William Gilbert: 1. 2. Electrification is not limited to amber; it’s a general phenomenon Amber (by wool) + feather Magnetite (Fe2O3) + iron - 700 0 Charles Dufay (King of France’s gardener): 1733 1600 Hans Oersted: Michael Faraday: Inverse-square force law for electricity Connection b/w electricity and magnetism (compass needle is deflected by current) 1. 2. Heinrich Hertz: Produced EM waves in the lab 1887 Concept of E & M fields EM Induction (changing magnetic field produces current in a circuit) 1831 1820 Alexander Popov Joseph Thomson: Guglielmo Marconi: Discovery of the Radio electron 1896 1. + and – electricity 2. Likes repel, opposites attract Electrically charged objects can also repel each other Charles Coulomb: 1785 Benjamin Franklin: 1897 1750 James Clerk Maxwell: 1. Laws of E&M in modern form 2. Existence of EM waves 3. Light is an EM wave 1865 to 1873 P. N. Lebedev: E. Rutherford: Niels Bohr: Light pressure “planetary” model of atom “(semi-) quantum” model of atom 1900 1911 1913 Michael Faraday (22 September 1791 – 25 August 1867), one of the most influential scientists in history. Discoveries include: • • • • • • Michael Faraday, 1842 The concept of the electromagnetic field Faraday's law of electromagnetic induction Electrochemistry (Faraday's laws of electrolysis; Faraday constant) Chemistry (discovered benzene, invented an early form of the Bunsen burner and the system of oxidation numbers, and popularized terminology such as anode, cathode, electrode, and ion.) Faraday effect (magnetic field causes a rotation of the plane of polarization of light - the first experimental evidence that light and electromagnetism are related) Faraday wheel (which formed the foundation of electric motor technology.) The SI unit of capacitance, the farad, is named in his honor. +1 +6 -2 H2SO4 Albert Einstein kept a picture of Faraday on his study wall, alongside pictures of Isaac 46 Newton and James Clerk Maxwell. 47 The Field Concept (1) 1) Taken literally, Coulomb’s Law describes an Action-at-a-Distance Model of electrostatic interactions in which charges (charged particles) exert forces directly and instantaneously on one another across the distance separating them. Note: these forces act along the lines connecting the charges Symbolically: charge charge 2) This model is good when charges are at rest. 3) Problems arise when charges are allowed to move. Example: - Charge 1 on Earth, charge 2 on Moon. - If charge 1 wiggles (for whatever reason), charge 2 (according to Coulomb) would immediately experience a different force. - This doesn’t seem right! - This leads to violation of STR, according to which no influence can propagate faster than the speed of light. 48 The Field Concept (2) 1) The Field Model instead imagines that a charge particle creates a field in the space around it, and another particle responds to the field at its own location, not to the first particle directly. Symbolically: charge field charge 2) How does this resolve the problem of moving charges? In our previous example: - When charge 1 is wiggled, it does not directly affect the distant charge 2. - Rather, the wiggling particle wiggles the values of the field in its immediate vicinity. - These wiggles in turn affect the field values at slightly more distant locations, and so on. - The net effect is that ripples in the field move away from the wiggling particle at a finite speed (similar to how ripples on the surface of water do; the difference is, the ripples in the field do not need any medium to propagate in, so they can propagate in a vacuum). - As a result, only when the ripples reach the distant charged particle will it feel a wiggling force. The Field Concept (3) 1) A field, (unlike a particle) exists not at a specific location but throughout space. 2) Even so, the field is a physical object (entity) that (like a particle) has energy, carries momentum, and obeys its own equations of motion. 3) We need a field model b/c instantaneous action at a distance violates STR (no signal can propagate faster than the speed of light). The Field Model naturally resolves this problem. 4) Mathematically, we describe a field (formally) by assigning some kind of numerical quantity to every point in space at every moment in time – in our case, vectors. 5) Physically, we define the field (operationally) in terms of what it does – in our case, in terms of forces it exerts on charged particles. The Field Concept (4) 1) So, physically, we define the field (operationally) in terms of what it does – in our case, in terms of forces it exerts on charged particles. 2) Here’s how it works: - + + -- -+ +-+-+ + +- P Bring in qtest and hold it at rest; then measure the force on it. 3) Then, by definition: Distribution of charge (regarded as the source of the field at point P). Charges in this distribution are allowed to move arbitrarily. Fe E≡ qtest By definition: The Field Concept (5) Fe E≡ qtest Translation: a) This eq. defines the E-field vector at a point in space & time. b) Fe is the electrostatic force experienced at that time by a small test particle with charge qtest held at rest at that point in space. c) qtest must be small, so that the force it exerts on the charges in the distribution does not push around the charges whose field we are trying to measure. d) E-vector points in the same direction as Fe if qtest is positive. e) Why divide Fe by qtest? – B/c it is found experimentally that, no matter how source charges move, the force Fe the test charge experiences at a given location is proportional to qtest itself. So, dividing by qtest produces a quantity E that depends only on position relative to the charges creating the field and not on the magnitude of the test charge qtest we use. f) Why keep qtest at rest? – B/c if qtest moves (has non-zero velocity) it will experience an additional force (magnetic force) due to motion of the source charges. By definition: Note: The Field Concept (6) Fe E≡ qtest E-field is a vector quantity, but it is important to remember that it consists of an infinite number of vectors attached to every point in space at any moment in time. To describe the E-field fully you must specify E-vectors everywhere. Unit: [E] = N/C Examples: 1. On a sunny day, due to various atmospheric processes that separate charges, E = 100 to 150 (N/C) 2. During a thunderstorm, E > 10,000 (N/C) 3. When taking a shower, by moving water, E ~ 800 (N/C) 4. In dry air, if E > 3,000,000 (N/C), the air breaks and becomes a conductor, sparks fly. The Field Concept (7) Once the E-field has been determined, we can find the force it exerts on any charged particle by: Fe = qE (any charge; limitations apply) Note: Fe E (if q > 0) Fe E (if q < 0) 55 56 (magnitude) 57 Electric field lines. 59 60 61 Electric dipole. 62 63 Approx. value; found without a calculator. 64 65 The following is not needed in our class. 66 Gauss’ Law 67 68 69 70 71 72 Gauss’ Law (concepts) 73 74 75 E2 E1 All of these guesses are wrong! 76 E1 E2 77 Gauss’ Law (extra slides from previous semester; not really needed) 78 79 80 81 82 83 84 85 86 A voltaic pile (~1800) on display in the Tempio Voltiano. 87 More slides from previous semester (not really needed) 88 89 90 91 92 93 94 95 The End 96