* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Poster
DNA vaccination wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Adaptive immune system wikipedia , lookup
Innate immune system wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
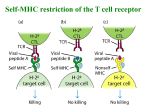
“You Look Familiar…” How the immune system specifically targets and kills infected cells Homestead SMART Team: Rahul Subramanian, Sahitya Raja, Nikhil Ramnarayan, Sophia Dantoin, Dhruv Metha, Yelena Ostrerov Instructor: Ms. Chris Schultz Mentor: Andrea Ferrante, MD, BRI - BloodCenter of Wisconsin Abstract The Acquired Immune System offers highly specific protection from infection by viruses, bacteria and other microbes by recognizing the pathogen, triggering an immune response resulting in pathogen elimination, and establishing immunological memory for future recognition. A particular type of response involves T-lymphocytes, which play regulatory and/or killing functions. In particular, cytotoxic Tlymphocytes (CTL) identify and kill infected cells by examining pieces of proteins (antigens) on the cell membrane as here illustrated by the CTL response to the influenza virus. Each CTL scans the antigen repertoire with its receptor (TCR), and upon binding with a cognate complex, destroys the infected cell. 1. Infection Structural Considerations Host Cell Viral peptide pieces Influenza Virus In the model pictured below, due to the specific recognition by the TCR JM22 (left) of the viral peptide MP 58-66 presented within the Class-I HLA-A2 protein (right) , the host cell will be killed. MHC A cell is infected by an influenza virus. A viral peptide is transported to the ER, where it is encased in an MHC Class-I presentation protein. 2. Presentation We present the outstanding model of an influenzaderived peptide (MP (58-66)) bound to HLA-A2 (a membrane molecule which presents antigens to T cells), and complexed with the TCR Vβ17Vα10.2. Host Cell MHC 4. Killing and apoptosis PDB ID 1OGA.pdb The MHC- peptide complex is transported to the cell membrane, where the MHC presents the peptide. Conclusion The CTL response to the HLA-A2/MP(58–66) complex can be an instructive model of immune memory to an antigen of a frequently encountered virus that usually is cleared from the body. Interestingly, this response mainly recruits T cells bearing particular TCR-α and β chains. TCR Vβ17 accounts for between 55 and 85% of all β chains used in this response, and we showed the structural characteristics giving reason to this phenomenon. Moreover, the observation here presented suggests that this domain may have coevolved with the HLA-A2 to respond to the strong selection pressure of recurrent and often lethal influenza pandemics. Upon recognition of the peptide, the T cell immediately destroys the host cell. If the T cell fails to recognize the cell, it continues to examine other cells in the area. T cell TCR MHC Host cell 3. Recognition A T cell able to recognize a specific peptide sequence scans the peptide using its T Cell Receptor. A SMART Team project supported by the National Institutes of Health (NIH) – National Center for Research Resources Science Education Partnership Award (NCRR-SEPA) Each chain of the TCR possesses one variable (V) domain, one constant (C) domain, a transmembrane/cell membranespanning region, and a short cytoplasmic tail at the C-terminal end. The final structure is the outcome of a rearrangement process at the DNA level during which one gene segment among several encoding for each domain is randomly selected and joined with the others. In our model, there are four crucial hotspot residues for TCR binding. Of these, three are encoded by a TCR gene sequence known as V β (β chain) 17 (gene segment number) which is stable through T cell development. The fourth is encoded by a high number of codons in a gene portion which is modified during cell development. It seems plausible that the number of T cells with Vβ17 receptors which are capable of binding the HLA-A2flu is higher than other HLA-A2-fluspecific TCRs, and may explain the dominance of this CTL response. Depletion of T cells with Vβ17 receptors from a cell culture dramatically decreases the ability of the remaining T cell population to kill fluinfected cells References P.J. Lehner, E.C. Wang, P.A. Moss, S. Williams, K. Platt, S.M. Friedman, J.I. Bell and L.K. Borysiewicz, Human HLA-A0201restricted cytotoxic T lymphocyte recognition of influenza A is dominated by T cells bearing the V beta 17 gene segment, J. Exp. Med. 181 (1995), pp. 79–91 Stewart - Jones, Guillaume B E, Andrew J. McMichael, John I. Bell, David I. Stuart, and Yvonne E. Jones. "A Structural Basis for Immunodominant Human T Cell Receptor Recognition." Nature Immunology (2003): 1-4.