* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Poster
Survey
Document related concepts
Transcript
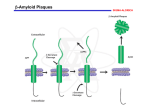
Don’t Forget It: Thrombin Related Alzheimer's Disease Lincoln SMART Team: Eric Auchter, Vanessa Heck, Danielle Niquette, Jenna Schuh, Kira Schultz, Jen Stenson, Brandon Vance Teacher: Mrs. Ann Hansen, Lincoln High School, Manitowoc, WI Mentors: Dr. Sally Twining, Professor and Malathi Narayan, Graduate Student, Department of Biochemistry Medical College of Wisconsin, Milwaukee, WI Abstract II. Alzheimer’s disease is an incurable and terminal neurodegenerative disorder and is the most common form of dementia. There are 5.2 million people in the United States living with Alzheimer’s and it is projected that 10 million baby boomers will eventually develop the disease. One of the three major competing hypotheses explaining Alzheimer’s involves hyperphosphorylation, the addition of more than one phosphate group, to tau, an intracellular microtubule protein. Thrombin is a serine protease involved in the final step of blood coagulation and is normally present in the brain’s neurons, where it cleaves tau. It is necessary for thrombin to cleave tau in order to prevent clumping of the microtubules for normal neuron function. In Alzheimer’s patients, extracellular thrombin binds and cleaves PAR-1, a protease activated receptor embedded in the cell membrane. The interaction between thrombin and PAR-1 exposes a peptide sequence that activates kinase enzymes adding phosphate groups to tau. This leads to tau hyperphosphorylation and tangled microtubules because intracellular thrombin is unable to cleave the phosphorylated tau. Scientists are interested in studying the binding of thrombin to PAR-1 because this interaction is a possible therapeutic target for developing treatments for Alzheimer’s and other neurodegenerative disorders. I. Introduction Normal vs. Alzheimer’s Disease Thrombin-Tau Interactions Tau, a microtubule associated protein, is normally cleaved by intracellular thrombin. This results in ordered and stabilized microtubules and normal neuron function. Normal 3bef.pdb Alzheimer's Disease Membrane Kinase PAR-1 Extracellular thrombin attaches to PAR-1 and cleaves the N-terminal peptide. III. The resulting exposed peptide sequence binds PAR-1 and activates kinase enzymes. Phosphate group Kinase enzymes add phosphate groups to tau. Laboratory Evidence: Effect of Thrombin on Tau Hypothesis: Excess thrombin results in tau aggregation. Experimental Procedure: 1. Nerve cells are grown on a slide. 2. The primary antibody to tau is added and binds each cell. Alzheimer's disease affects the elderly, causing memory loss and dementia. Thrombin, a serine protease, is found in high levels in the brains of Alzheimer’s disease patients. There it binds PAR-1, a membrane embedded receptor, and triggers a multistep process that leads to the tangling of a microtubule protein, tau. The resulting tau tangling is one hypothesis that explains the destruction of neurons and progression of Alzheimer’s disease. Microtubules form support structures for neurons and assist in transporting substances into and out of the cells. 3. A secondary antibody containing a fluorescent label is added and binds to the primary antibody. Data: Antibodies to: Total Tau Phosphorylated Tau Conclusion: • Without thrombin, tau is not phosphorylated, as shown by the absence of fluorescent spots in the top row. • In the presence of thrombin, tau is phosphorylated, leading to tangling, as shown in the bottom row. IV. Thrombin is unable to cleave phosphorylated tau because the phosphorylated residues mask the sites normally recognized by thrombin. Tangled microtubules result in abnormal neuron function. Summary Thrombin, a protein normally found in the brain’s neurons, is present outside the brain cells in Alzheimer’s disease. Thrombin cleaves the PAR-1 receptor and initiates a signaling process that eventually results in hyperphosphorylation of tau. Intracellular thrombin, normally responsible for cleaving tau, is unable to do so. The resulting tau tangling progressively destroys the brain’s neurons and leads to death. References: • Gandhi, P.S., Chen, Z., Mathews, F.S., Di Cera, E. (2008). Structural Identification of the Pathway of Long-range Communication in an Allosteric Enzyme. Proceeding of the National of Academy of Science USA 105: 18321837 • www.noelderabuse.com (Section 1 Photograph) • www.afar.com (Section 2 Photograph) • Suo, Z., Wu, M., Citron, B.A., Palazzo, R.E., Festoff, B.W. (2003). Rapid Tau Aggregation and Delayed Hippocampal Neuronal Death Induced by Persistent Thrombin Signaling. The Journal of Biological Chemistry, ASBMB (Section 3 Photograph) • 3BEF.pdb Gandhi, P.S., Chen, Z., Mathews, F.S., Di Cera, E. (2008) Structural identification of the pathway of long-range communication in an allosteric enzyme. Proc.Natl.Acad.Sci.Usa 105: 1832-1837 A SMART Team project supported by the National Institutes of Health (NIH) – National Center for Research Resources Science Education Partnership Award (NCRR-SEPA)