* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Activities for the -Helix and -Sheet Construction Kit
Fatty acid metabolism wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Butyric acid wikipedia , lookup
Catalytic triad wikipedia , lookup
Citric acid cycle wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Homology modeling wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Point mutation wikipedia , lookup
Metalloprotein wikipedia , lookup
Proteolysis wikipedia , lookup
Peptide synthesis wikipedia , lookup
Genetic code wikipedia , lookup
Amino acid synthesis wikipedia , lookup
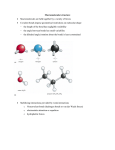
Activities for the α-Helix / β-Sheet Construction Kit The primary sequence of a protein, composed of amino acids, determines the organization of the sequence into the secondary structure. There are two periodic secondary structure motifs, α-helix and β-sheet. Proteins can be composed of primarily α-helices (for example, β-globin) or β-sheets (for example, GFP), or a combination of the two structural motifs. The construction kits allow students to interact with these secondary structures to explore similarities and differences between the two types. Teaching Points: • • • • • Each amino acid is similar, but different. The backbone of the amino acid is the same, but the sidechain (R group) is different. The unique identity of each amino acid is determined by the structure of the sidechain (R group). Sidechains have specific chemical properties determined by the chemical composition of the group. There are two repeating secondary structure motifs: α-helix and β-sheet. The secondary structure is dependent on phi-psi angles of the backbone peptide pieces. Activities for the α-Helix/β-Sheet Construction Kit Page 1 of 5 I. Backbone Pieces There are two versions of the α-helix / β-sheet construction kit. Blank/Green A (antiparallel) Green/Yellow Dots A. Examine one α-helix backbone piece and one β-sheet backbone piece (there is an A or a yellow dot located on the backbone α-carbon atom of the β-sheet piece.) 1. How are the two pieces similar? 2. How are the two pieces different? B. The backbone pieces are colored with CPK coloring: Carbon (gray) Oxygen (red) Nitrogen (blue) 1. Draw the chemical structure of the backbone model. 2. Identify the α-carbon. What bonds to the α-carbon? 3. What part of the amino acid structure is missing in this representation? C. Examine the orientation of the angle formed by the bond between the nitrogen and the α-carbon and the bond between the α-carbon and the carbonyl-carbon in each backbone piece. 1. How are these angles different? 2. Why are the angles important for building secondary structure? Activities for the α-Helix/β-Sheet Construction Kit Page 2 of 5 II. Building an α-Helix or a β-Sheet A. Build an α-helix and a β-sheet without any sidechains and without any hydrogen bonds. Examine the two secondary structures. 1. What similarities are present between the two structures? 2. What differences are present between the two? 3. How is the amino-group of one amino acid positioned in relation to other amino groups? The carboxyl carbons? B. Add the hydrogen bonds to the structure. 1. Where do hydrogen bonds form within each secondary structure? 2. What function do these bonds have on the overall structure? C. Measure the length of ten amino acids in an α-helix and ten amino acids in a β-sheet (two strands of five amino acids). 1. Which secondary structure is longer? 2. Why? 3. What impact might this difference have on the structure/shape of the protein? D. Mix the two types of backbone pieces together and build a structure. 1. Were you able to successfully build a secondary structure? 2. Why or why not? Activities for the α-Helix/β-Sheet Construction Kit Page 3 of 5 III. The Unique Identity of Each Amino Acid is Determined by the Chemical Composition of the Sidechain (R Group) There are 20 amino acids that serve as the building blocks of proteins. Each amino acid has the same backbone (NH2-CHRCOOH). The identity of the amino acids is determined by the chemical composition of the R group. We can group the sidechains according to chemical properties, such as hydrophobic (non-polar) versus hydrophilic (polar). A. Exploring the Sidechains There are 30 sidechains in each kit. There are multiple copies of several amino acids. 1. Which amino acids have two copies? 2. Which amino acids have three copies? 3. Which amino acid has four copies? 4. Which amino acid is not included in this selection of sidechains? 5. Why do you think this amino acid is not included? B. Examine each amino acid. 1. Based on the chemical nature of each sidechain, how can you organize these sidechains into groups? 2. What similarities/differences do you see between a. Tyrosine and phenylalanine b. Serine and threonine c. Glutamine and glutamic acid d. Asparagines and aspartic acid e. Leucine and isoleucine f. Valine and leucine g. Alanine and valine h. Methionine and cysteine i. Lysine and arginine j. Tryptophan and phenylalanine k. Histidine and tryptophan 3. Looking at your structures of the α-helix and the β-sheet, which sidechains do you think would be better suited for each type of secondary structure? Activities for the α-Helix/β-Sheet Construction Kit Page 4 of 5 IV. Putting it Together A. Create a tetra-peptide using the backbone pieces (either the α-helix or the β-sheet) and sidechains. 1. Draw the chemical structure of your tetra-peptide. 2. Identify the peptide bond on your drawing. 3. Identify the sidechain (R group) on your drawing. B. Modeling Helix A of the β-Globin Protein (please see the handout that accompanies the α-Helix/β-Sheet Collection) C. Modeling β-Sheet of the Green Fluorescent Protein (please see the handout that accompanies the α-Helix/β-Sheet Collection) **Possible misconceptions to be aware of when using this kit: Amino acids are not assembled by backbone pieces first and then the addition of the sidechains. The sidechains are separate in this kit to allow for exploration of the different structures of sidechains and to allow for constructing models of the α-helix and β-sheet backbones structures. Copyright © 1998 - 2008 Center for BioMolecular Modeling. All rights reserved. Revised 11/12 Activities for the α-Helix/β-Sheet Construction Kit Page 5 of 5