* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Absorption, distribution, metabolism and excretion
Compounding wikipedia , lookup
Discovery and development of cephalosporins wikipedia , lookup
Orphan drug wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Discovery and development of cyclooxygenase 2 inhibitors wikipedia , lookup
Theralizumab wikipedia , lookup
Drug design wikipedia , lookup
Drug discovery wikipedia , lookup
Psychopharmacology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
Plateau principle wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Neuropharmacology wikipedia , lookup
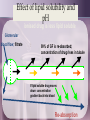
Absorption, distribution, metabolism and excretion • relevant to ALL drugs • large research/development area • frequent cause of failure of treatment – failure of compliance – failure to achieve effective level – produce toxic effects – drug interactions (*******) • can enhance patient satisfaction with treatment • understand different dosage forms available Learning objectives • To obtain a broad perspective over the field of ADME for drug molecules as a whole; • To understand why ADME is important to drug action; • To learn about the major pathways and mechanisms involved in ADME of drugs; • To understand the importance of ADME to the dental practitioner; • To obtain a basic knowledge about the quantitative aspects of ADME, pharmacokinetics Overview - ADME Most drugs : enter the body (by mouth or injection or…) - must cross barriers to entry (skin, gut wall, alviolar membrane…..) are distributed by the blood to the site of action - intra- or extracellular - cross barriers to distribution (capillaries, cell wall….) - distribution affects concentration at site of action and sites of excretion and biotransformation are biotransformed perhaps to several different compounds by enzymes evolved to cope with natural materials - this may increase, decrease or change drug actions are excreted (by kidney or …….) which removes them and/or their metabolites from the body Pharmacokinetics is the quantification of these processes Overview - ABSORPTION Some drugs work outside the body (barrier creams, some laxatives) but most must: • enter the body: Given by: ENTERAL - oral, sublingual, buccal, rectal PARENTERAL sc, im, iv, it • cross lipid barriers / cell walls: gut wall, capilliary wall, cell wall, blood brain barrier ---- get into the body and (after distribution) to reach the cellular target ---- Passage through lipid membranes • diffusion through gaps between cells (glomerulus = 68K; capillary 30K; NB brain capillary - tight junction) • passage through the cell membrane – diffuse through pore (very small; use dependent) – carrier mediated transport (specific, saturable; Fe in gut; LDOPA at blood-brain barrier) – pinocytosis (insulin in CNS; botulinum toxin in gut) – diffusion through lipid of cell membrane (depends on AREA, DIFFUSION GRADIENT, DIFFUSION COEFFICIENT, LIPID SOLUBILITY) Ion channel Carrier Pinocytosis Diffusion Lipid solubility :weak acids and weak bases HA <==> H+ + A[ UI ] [I] pKa=pH+log(HA/A-) ASPIRIN pKa = 4.5 (weak acid) 100mg orally 0.1 = [ I ] Stomach pH = 2 99.9 = [ UI ] Blood pH = 7.4 [ UI ] Aspirin is reasonably absorbed from stomach (fast action) B + HCl <==> BH+ + Cl[ UI ] [I] pKa=pH+log(BH+/B) STRYCHNINE pKa = 9.5 (weak base) 100mg orally 99.9 = [ I ] Stomach pH = 2 0.1 = [ UI ] Blood pH = 7.4 [ UI ] Strychnine not absorbed until enters duodenum Routes of administration • Enteral; oral, sub-lingual (buccal), rectal. Note soluble, enteric coated or slow release formulations • Parenteral; iv, im, sc, id, it, etc. Different rates of absorption, different plasma peaks. Note iv infusors • • • • Skin; for local or systemic effect - note patches Lungs; inhalation; local or systemic effect? Vaginal; (usually local) Eye; (usually local) Factors affecting oral absorption • • • • • • • • • • Disintegration of dosage form Dissolution of particles Chemical stability of drug Stability of drug to enzymes Motility and mixing in GI tract Presence and type of food Passage across GI tract wall Blood flow to GI tract Gastric emptying time FORMULATION Bioavailability • the proportion of the drug in a dosage form available to the body i.v injection gives 100% bioavailability. Calculated from comparison of the area under the curve (AUC) relating plasma concentration to time for iv dosage compared with other route. Says nothing about effectiveness. Bioavailability Destroyed in gut Dose Not absorbed Destroyed by gut wall Destroyed by liver to systemic circulation Bioavailability Plasma concentration 70 60 i.v. route 50 (AUC)o / (AUC)iv 40 oral route 30 20 Time (hours) 10 0 0 1 2 3 4 5 6 7 8 9 Plasma digoxin ng/ml Bioequivalence: steady-state digoxin: different maker’s products (0.25mg) 3.5 3 2.5 2 1.5 1 0.5 0 1 2 3 4 Maker 5 6 7 8 Sustained release preparations • depot injections (oily, viscous, particle size) • multilayer tablets (enteric coated) • sustained release capsules (resins) • infusors (with or without sensors) • skin patches (nicotine, GTN) • pro-drugs • liposomes Targeted drugs , antibody-directed Overview - DISTRIBUTION The body is a container in which a drug is distributed by blood (different flow to different organs) - but the body is not homogeneous. Note local delivery (asthma). • Volume of distribution = V = D/Co plasma (3.5 l); extracellular fluid (14 l); intracellular fluid (50 l); + special areas (foetus, brain) • note::: plasma protein binding tissue sequestration ----- brings drug to target tissue and affects concentration at site of action/elimination----- Distribution into body compartments • Plasma 3.5 litres, heparin, plasma expanders • Extracellular fluid 14 litres, tubocurarine, charged polar compounds • Total body water 40 litres, ethanol • Transcellular small, CSF, eye, foetus (must pass tight junctions) Plasma protein binding; Tissue sequestration; pH partition =1.5; antilog 1.5 = 31.6 1.6 logCt = logCo - Kel . t 2.303 1.1 0.6 TIME (hours) 0.1 0 5 -0.4 log plasma -0.9 concentration 10 15 20 Alter plasma binding of drugs 1000 molecules 99.9 % bound 90.0 1 molecules free 100 100-fold increase in free pharmacologically active concentration at site of action. Effective TOXIC Overview - METABOLISM • Drug molecules are processed by enzymes evolved to cope with natural compounds • Drug may have actions increased or decreased or changed • Individual variation genetically determined • May be several routes of metabolism • May not be what terminates drug action • May take place anywhere BUT liver is prime site • Not constant - can be changed by other drugs; basic of many drug-drug interactions … metabolism is what the body does to the drug Biotransformation of drugs • Mutations allowing de-toxification of natural toxic materials are advantageous and are selected • Drugs are caught up in these established de-toxification processes • Drugs may converted to less toxic/effective materials more toxic/effective materials materials with different type of effect or toxicity Sites of biotransformation • where ever appropriate enzymes occur; plasma, kidney, lung, gut wall and LIVER • the liver is ideally placed to intercept natural ingested toxins (bypassed by injections etc) and has a major role in biotransformation The liver Hepatocytes portal venous blood systemic arterial blood smooth endoplasmic reticulum bile microsomes contain cytochrome P450 dependent mixed function oxidases venous blood Types of biotransformation reaction • Any structural change in a drug molecule may change its activity • Phase I - changes drugs and creates site for phase II oxidation (adds O) eg. Microsomes (P450); reduction; hydrolysis (eg. by plasma esterases) others • Phase II - couples group to existing (or phase I formed) conjugation site glucuronide (with glucuronic acid) sulphate others OH Phase I Phase II O-SO3 Cytochrome P450 dependent mixed function oxidases DRUG METABOLITE =DRUG+O O2 microsome NADPH H+ NADP+ WATER There are several different types of mixed function oxidase - different specificity Genetic polymorphism in cytochrome P450 dependent mixed function oxidases CYP FOUR families 1-4 SIX sub-families A-F up to TWENTY isoenzymes 1-20 CYP3A4 : CYP2D6 : CYP2C9 : CYP2C19 :CYP2A6 CYP2D6*17 (Thr107Ile; Arg296Cys) Caucasian 0% Africans 6% Asian 51% - reduced affinity for substrates No. of patients 25 Plasma conc in 267 patients after 9.8 mg/kg isoniazid orally 20 15 10 5 0 0 1 2 3 Isoniazid conc. ug/ml 9 10 11 12 PHASE 1 reactions Hydroxylation -CH2CH3 Oxidation -CH2OH N-de-alkylation -N(CH3)2 -CH2CH2OH -CHO -COOH - NHCH3 + CH3OH Oxidative deamination -CH2CHCH3 -CHCOCH3 + NH3 | NH2 PHASE 2 reactions Conjugations with glucuronide, sulphate …. alters activity, made less lipid soluble so excreted PHASE 2 reactions (not all in liver) CONJUGATIONS • -OH, -SH, -COOH, -CONH with glucuronic acid to give glucuronides • -OH with sulphate to give sulphates • -NH2, -CONH2, aminoacids, sulpha drugs with acetyl- to give acetylated derivatives • -halo, -nitrate, epoxide, sulphate with glutathione to give glutathione conjugates all tend to be less lipid soluble and therefore better excreted (less well reabsorbed) Other (non-microsomal) reactions • Hydrolysis in plasma by esterases (suxamethonium by cholinesterase) • Alcohol and aldehyde dehydrogenase in cytosolic fraction of liver (ethanol) • Monoamine oxidase in mitochondria (tyramine, noradrenaline, dopamine, amines) • Xanthene oxidase (6-mercaptopurine, uric acid production) • enzymes for particular drugs (tyrosine hydroxylase, dopa-decarboxylase etc) Phase I in action Imipramine 4-hydroxy imipramine (cardiotoxic) N CH2 CH2 N CH3 CH3 desmethyl imipramine (antidepressant) Factors affecting biotransformation • race (CYP2C9; warfarin (bleeding) phenytoin (ataxia) Losartan (less cleared but less activated as well); also fast and slow isoniazid acetylators, fast = 95% Inuit, 50% Brits, 13% Finns, 13% Egyptians). • age (reduced in aged patients & children) • sex (women slower ethanol metabilizers) • species (phenylbutazone 3h rabbit, 6h horse, 8h monkey, 18h mouse, 36h man); biotransformation route can change • clinical or physiological condition • other drug administration (induction (not CYP2D6 ) or inhibition) • food (charcoal grill ++CYP1A)(grapefruit juice --CYP3A) • first-pass (pre-systemic) metabolism Inhibitors and inducers of microsomal enzymes • INHIBITORS cimetidine prolongs action of drugs or inhibits action of those biotransformed to active agents (pro-drugs) • INDUCERS barbiturates, carbamazepine shorten action of drugs or increase effects of those biotransformed to active agents • BLOCKERS acting on non-microsomal enzymes (MAOI, anticholinesterase drugs) The enterohepatic shunt Drug Liver Bile formation Bile duct Hydrolysis by beta glucuronidase Biotransformation; gall bladder glucuronide produced Portal circulation Gut Overview - EXCRETION • Urine is the main but NOT the only route. • Glomerular filtration allows drugs <25K MW to pass into urine; reduced by plasma protein binding; only a portion of plasma is filtered. • Tubular secretion active carrier process for cations and for anions; inhibited by probenicid. • Passive re-absorption of lipid soluble drugs back into the body across the tubule cells. Note effect of pH to make more of weak acid drug present in ionised form in alkaline pH therefore re-absorbed less and excreted faster; vica-versa for weak bases. Effect of lipid solubility and pH ionised drug is less lipid soluble Glomerular blood flow; filtrate 99% of GF is re-absorbed; concentration of drug rises in tubule If lipid soluble drug moves down concentration gradient back into blood Re-absorption Special aspects of excretion • lactating women in milk • little excreted in faeces unless poor formulation or diarrhoea • volatile agents (general anaesthetics) via lungs • the entero-hepatic shunt glucuronic acid conjugates with MW >300 are increasingly excreted in bile; hydrolysis of say -OH conjugate by beta-glucuronidase in gut will restore active drug which will be reabsorbed and produce an additional effect. Pharmacokinetics • Study of ADME on a quantitative basis In man study blood, urine, faeces, expired air. Measure urine volume & concentration of drug conc in urine x vol per min = RENAL plasma concentration CLEARANCE If neither secreted nor reabsorbed then clearance = clearance of inulin = 120 ml/min If completely cleared by secretion then clearance = clearance of p-hippuric acid = renal blood flow = 700 ml/min Plasma concentration 14 Ct = Co e-tKel lnCt = lnCo - Kel t 12 10 8 logCt = logCo - Kel . t 2.303 6 y 4 = c m x 2 0 0 2 4 6 8 10 12 TIME (hours) 14 16 18 20 1.6 =1.5; antilog 1.5 = 31.6 logCt = logCo - Kel . t 2.303 1.1 0.6 TIME (hours) 0.1 0 2 4 6 8 -0.4 -0.9 log plasma concentration 10 12 14 16 18 20 Pharmacokinetic parameters • Volume of distribution • Plasma clearance V = DOSE / Co Cl = Kel .V • plasma half-life (t1/2) or directly from graph t1/2 = 0.693 / Kel • Bioavailability (AUC)x / (AUC)iv Multiple dosing • In a dental context some drugs are given as single doses. Many however are given as a course of therapy • On multiple dosing plasma concentration will rise and fall with each dose and will increase until administration = elimination ie. steady state is reached. • At each dose the level will oscillate through a range • The objective is to remain within the therapeutic window, with acceptable variation at each dose and with a regimen which promotes compliance. plasma conc toxic 7 6 5 effective 4 Cumulation and use of loading doses 3 2 1 Time 0 0 5 10 15 20 25 30