* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Absorption, distribution, metabolism and excretion
Survey
Document related concepts
Transcript
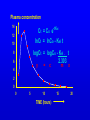
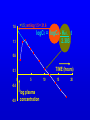
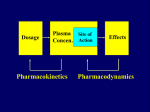
Learning objectives • To know what data is available from pharmacokinetic studies in man and how to handle it • To know how to calculate the basic pharmacokinetic parameters of clearance, t(half); volume of distribution, bioavailability; Kel • To understand the implications of these parameters for satisfactory therapy and the construction of suitable dosage regimens for patients • To know how knowledge of pharmacokinetic parameters can be exploited in drug design and formulation development. Pharmacokinetics • Study of ADME on a quantitative basis In man study blood, urine, faeces, expired air. Measure urine volume & concentration of drug conc in urine x vol per min = RENAL plasma concentration CLEARANCE If neither secreted nor reabsorbed then clearance = clearance of inulin = 120 ml/min If completely cleared by secretion then clearance = clearance of p-hippuric acid = renal blood flow = 700 ml/min Plasma concentration 14 Ct = Co e-tKel lnCt = lnCo - Kel t 12 10 logCt = logCo - Kel . t 2.303 8 6 y 4 = c m x 2 0 0 5 10 TIME (hours) 15 20 Bioavailability Plasma concentration 60 i.v. route 50 (AUC)o / (AUC)iv 40 30 oral route 20 Time (hours) 10 0 0 2 4 6 8 =1.5; antilog 1.5 = 31.6 1.6 logCt = logCo - Kel . t 2.303 1.1 0.6 TIME (hours) 0.1 0 5 -0.4 -0.9 log plasma concentration 10 15 20 Pharmacokinetic parameters • Volume of distribution V = DOSE / Co • Plasma clearance Cl = Kel .V • plasma half-life (t1/2) or directly from graph t1/2 = 0.693 / Kel • Bioavailability (AUC)x / (AUC)iv Multiple dosing • On multiple dosing plasma concentration will rise and fall with each dose andwill increase until administration = elimination ie. steady state is reached. • At steady state hourly dose rate (D=dose; T=interval between doses in hours) D/T = clearance x plasma conc or steady state conc = D/(T x clearance) • At each dose the level will oscillate through D/V plasma conc toxic 6 5 4 effective Cumulation and use of loading doses 3 2 1 Time 0 0 5 10 15 20 25