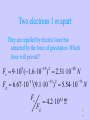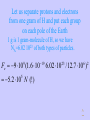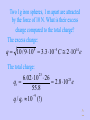* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download I-1
History of quantum field theory wikipedia , lookup
Casimir effect wikipedia , lookup
Introduction to gauge theory wikipedia , lookup
History of electromagnetic theory wikipedia , lookup
Magnetic monopole wikipedia , lookup
Standard Model wikipedia , lookup
Newton's laws of motion wikipedia , lookup
Elementary particle wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Anti-gravity wikipedia , lookup
Speed of gravity wikipedia , lookup
Work (physics) wikipedia , lookup
Maxwell's equations wikipedia , lookup
Centripetal force wikipedia , lookup
Field (physics) wikipedia , lookup
Electromagnetism wikipedia , lookup
Fundamental interaction wikipedia , lookup
Lorentz force wikipedia , lookup
PY212 Electricity and Magnetism I. Electrostatics 07. 07. 2003 1 I-1 Electric Charge 07. 07. 2003 2 Main Topics • • • • • • Why Electrostatics? Demonstration of Electrostatic Effects. Electric Charge and its Properties. Coulomb’s Law. Some Applications of the C. L. Electric Field and Electric Intensity 07. 07. 2003 3 Why Electrostatics? • Many important properties of the Nature exist due to electric interactions of charged particles. • We shall first deal with fields and charges which are static = do not move. • It is for simplicity but such fields really exist, if some equilibrium can be reached! 07. 07. 2003 4 Demonstration of Electrostatic Effects • A comb after it has been run through hair attracts little pieces of paper. The force is a long-distance one. It can be attractive or repulsive. • We attribute these forces to the existence of a property we call the electric charge. • Bodies can be charged by conduction via contact with other bodies but even remotely by induction. • Using some materials we can easily discharge charged bodies, these are conductors. By others it is slow or even impossible, they are insulators 07. 07. 2003 5 Main Properties of Charges • Since both the attractive and the repulsive forces exist, charges must be of two kinds, positive and negative. Unlike charges attract and like charges repel themselves. • Charges are quantized. They can only be isolated in integer multiples of the elementary charge e = 1.602 10-19 C • In all known processes charges appear or disappear only in pairs (+q and -q), so the total charge is conserved • Charge is invariant to the Lorentz transformation 07. 07. 2003 6 Main Properties of Electrostatic Interactions • Charged particles act by a force on each other. Forces : • are Long-distance – mediated by electric field • obey the principle of superposition • Mutual interaction of two static point charges is described by Coulomb’s Law 07. 07. 2003 7 Coulomb’s Law I • Let us have two point charges Q1 and Q2 at the distance r apart. Then the magnitude of the interaction force is: F = k Q1 Q2 / r2 • • • • The SI unit of charge is 1 Coulomb [1 C] k = 9 109 Nm2/C2 k = 1/40 0= 8.85 10-12 C2/ Nm2 is the permitivity of vacuum 07. 07. 2003 8 Coulom’s Law II • Since forces are involved the directional information is as important as the magnitude. • To get the full information let’s place Q1 into the origin and let describe the position of Q2 by the radius vector r . Then the force acting on Q2 is : kQ1Q2 r kQ1Q2 0 F21 (r ) r 2 2 r r r • Forces act in the straight line joining the charges . • Positive force is repulsive . • Forces acting on Q1 and Q2 are action and reaction of each other . 07. 07. 2003 9 Coulomb’s Law III • The most general formula we get if we define the position of each charge Qi (i=1, 2) by its own radius vector ri . Then the force acting on Q2 is : kQ1Q2 (r2 r1 ) F21 (r2 ) 3 | r2 r1 | • Since the force depends only on the difference between the radius vectors, the position of the origin is arbitrary . 07. 07. 2003 10 Comparison with the Force of Gravity • Formally, Coulomb’s Law is Analogous to Newton’s Gravitational Law • But electrostatic force is ~ 1042 (!) times stronger • Such a weak force still dominates the universe because matter is usually neutral • Charging something means to break very slightly the great equilibrium 07. 07. 2003 11 The Concept of the Field • If a charge is located in some point in the space it sends around an information about its position, polarity and magnitude. The information spreads with the speed of light. It can be “received” by another charge . The interaction of a charge with field produces a force. 07. 07. 2003 12 Electric Intensity I • Electrostatic field could be described by taking some test Q and recording the vector of the charge force F (r ) acting on it in every point of interest. • This description would, however, depend on the magnitude and polarity of the test charge and these properties would have to be provided as the additional information to make the description unique. 07. 07. 2003 13 Electric Intensity II • By dividing of the force by the magnitude of the test charge the electric intensity is defined : F (r ) E (r ) Q • It is a unique property of the described field, now. • Numerically it is equal to the force which would act in the particular point on a unit positive charge, but be careful with dimensions. 07. 07. 2003 14 Electric Intensity III • It is important to realize that by dividing by the magnitude of the charge, the information, how the charge ‘feels’ the field, becomes an objective property of the field. • The same field acts on various charges by various forces which can be even opposite due to the existence of two polarities of charges. 07. 07. 2003 15 Electric Field Lines I • Electric field is a three dimensional vector field which is in general case difficult to visualize. • In cases of simple symmetry, it is possible to use electric field lines which are lines tangent to vectors of the electric intensity in every point. The magnitude of the field can be illustrated by their length or density. 07. 07. 2003 16 Electric Field Lines II • A positive charge of a very little mass would move along a certain field line in the direction of the electric field while negative charge would move in the opposite direction. • Field lines can’t cross! 07. 07. 2003 17 Homework 1 • The homework is selected from “problem” sections that are in the end of each chapter. • Due Wednesday ! • 21- 2, 7, 9, 14, 15 07. 07. 2003 18 Things to Read : • This lecture covers: Giancoli: Chapter 21, Sections 1-8 (ex. 7) • Advance reading : Giancoli: 07. 07. 2003 Chapter 22 19 Two electrons 1 m apart They are repelled by electric force but attracted by the force of gravitation. Which force will prevail? Fe 9 10 (1.6 10 9 Fg 6.67 10 11 19 2 ) 2.31 10 (9.1 10 Fe 31 2 28 N ) 5.54 10 71 N 4.2 10 !!! 42 Fg ^ One electron and one proton 0.53 10-10 m apart This corresponds to their distance in hydrogen atom. Fe 9 10 (1.6 10 9 8.2 10 8 19 / 0.53 10 10 2 ) N Force of this magnitude can be in principle measured macroscopically! This is the secret why matter holds together. ^ Let us separate protons and electrons from one gram of H and put each group on each pole of the Earth 1 g is 1 gram-molecule of H, so we have NA=6.02 1023 of both types of particles. Fe 9 10 (1.6 10 9 19 6.02 10 / 12.7 10 ) 23 6 2 5.2 10 N (!) 5 ^ Two 1g iron spheres, 1 m apart are attracted by the force of 10 N. What is their excess charge compared to the total charge? The excess charge: 5 q 10 / 9 10 3.3 10 C 2 10 e 9 14 The total charge: 6.02 10 26 23 qt 2.8 10 e 55.8 9 q / qt 10 (!) 23 ^