* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download chapter-27-1-with
Chemical bond wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Tight binding wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Bremsstrahlung wikipedia , lookup
Particle in a box wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Ferromagnetism wikipedia , lookup
Elementary particle wikipedia , lookup
X-ray fluorescence wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Atomic orbital wikipedia , lookup
Electron configuration wikipedia , lookup
Atomic theory wikipedia , lookup
Matter wave wikipedia , lookup
Double-slit experiment wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
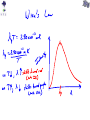
CH 26-1 Explaining a Continuous Spectrum (called a blackbody spectrum) First Evidence of Quantum Behavior: Blackbody Radiation Hot objects glow (toaster coils, light bulbs, the sun). As the temperature increases the color shifts from Red to Blue. The classical physics prediction was completely wrong! (It said that an infinite amount of energy should be radiated by an object at finite temperature.) Planck’s Quantum Hypothesis • First evidence of “quantum” behavior. Max Planck found he could explain these curves if he assumed that electromagnetic energy was radiated in discrete chunks, rather than continuously. The “quantum” of electromagnetic energy is called the photon. Energy carried by a single photon is E = hf = hc/l Planck’s constant: h = 6.626 X 10-34 J·s Wien’s Law (Example) The peak wavelength of light from Sun is at about 502 nm. What is the temperature of the Sun (specifically the photosphere of the Sun)? Poll Bulb A’s filament is reddish yellow. Bulb B’s filament is white. Which bulb’s filament has a greater temperature? 1. Bulb A 2. Bulb B 3. Neither; they have the same temperature. Photoelectric Effect • Light shining on a metal can “knock” electrons out of atoms. • Light must provide energy to overcome electrical attraction of electron to nucleus – This amount of energy is called the work function (W0) of the metal. Light on Metal: Wave Predictions • Intensity of light is related to strength of electric field in the electromagnetic wave. – Increasing intensity means the light wave can apply a stronger force to the electron. – Increasing intensity means the kinetic energy of the ejected electrons will increase. • Frequency of light is related to how fast the electric/magnetic fields oscillate. – Increasing the frequency does not change the force applied to electrons and so it will not change the kinetic energy of the ejected electrons. Max Kinetic Energy of ejected electrons Photoelectric Effect (Example) A metal whose work function is 1.5 eV is illuminated with 600 nm wavelength light. Will any electrons be ejected from the metal? If so, what is the maximum possible kinetic energy of the ejected electrons? • Wave Is Light a Wave or a Particle? – Electric and Magnetic fields act like waves – Superposition, Interference and Diffraction • Particle – Photons – Collision with electrons in photo-electric effect Both Particle and Wave ! Are Electrons Particles or Waves? • Particles, definitely particles. • You can “see them”. • You can “bounce” things off them. • How would we know if electron was a wave? Look for interference! Young’s Double Slit w/ electron d Source of monoenergetic electrons L 2 slitsseparated by d Screen a distance L from slits Electrons are Waves? • Electrons produce interference pattern just like light waves. – Need electrons to go through both slits. – What if we send 1 electron at a time? – Does a single electron go through both slits? Electrons are Particles and Waves! • Depending on the experiment electron can behave like – wave (interference) – particle (localized mass and charge) De Broglie Waves So far only photons have wavelength, but De Broglie postulated that it holds for any object with momentum- an electron, a nucleus, an atom, a baseball,…... Explains why we can see interference and diffraction for material particles like electrons!! Matter Waves (Example) Compare the wavelength of an electron with that of a two ton truck if each are moving at 30 m/s. me = 9.11×10−31 kg 2 tons = 909 kg