* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Immunologic Targeting - How to Channel a Minimal Response
Monoclonal antibody wikipedia , lookup
Duffy antigen system wikipedia , lookup
Major histocompatibility complex wikipedia , lookup
Adaptive immune system wikipedia , lookup
Sjögren syndrome wikipedia , lookup
DNA vaccination wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Molecular mimicry wikipedia , lookup
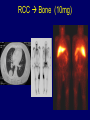
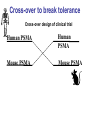
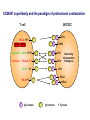
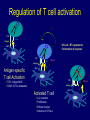
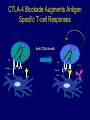
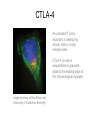
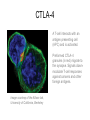
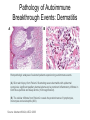
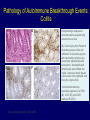
Immunologic Targeting - How to Channel a Minimal Response for Maximal Outcome Susan Slovin, MD, PhD Genitourinary Oncology Service December 1, 2005 Have we succeeded or failed in our treatments for prostate cancer? Success Failure Docetaxel – standard of care Not that many approved Ph III drugs Multiple targeting pathways Which is the “one” to stop growth New drugs in the pipeline Too many approved too fast or too few reaching approval status Responses after 1st line CAB Disease moves too fast Bone seeking drugs: improved toxicity profile No impact on important measures RATIONALE FOR TARGETED INTERVENTION PRO CON 1. Over-expression and 1. Strictly extracellular, no undergycosylation of contact with intracellular cell surface molecules pathways. Expression varies. 2. Can target receptor-like 2. Cell can develop “collateral” molecules which can signaling/survival pathways. stimulate intracellular 3. Cell can overcome via signaling pathways. multiple mechanisms 3. Can prevent target rendering tx inadequate. activation. TARGETS UNDER EXPLORATION • • • • Cell Surface Mucins, glycolipids, carbohydrates, glycoproteins [PSA, PSMA, KSA] AR EGFr Laminin • • • • • • Intracellular Vitamin D (calcitriol) HSP-90 Proteasome DNA (HDACs) BCL-2 [α-sense] Other – Stroma, neovasculature What have we learned from MSKCC prostate cancer vaccine trials? 1) chemical mimes of known cell surface molecules were shown to be immunogenic, ie Globo H - first time that a synethetic molecule could break immunologic tolerance in man 2) role of carriers such as KLH and adjuvants such as QS21 in enhancing immunogenicity and facilitating the immune response. QS21 still remains the best adjuvant through all clinical trials. 3) increasing doses of vaccine do not correlate with augmentation of immunogenicity, ie, lower doses appear to be more immunogenic (especially seen in the TF trial) 4) immunologic responses were not immediate but took up to 6 or more months to develop after the last vaccine; no role for boosters unless they were given either every 4-8 weeks. 5) we learned about the use of PSA slopes - no major impact on pts with high risk disease destined to progress within two years. 6) No clear cut immunologic endpoint; controversy as to how to design biologic trials – lead to PSA Working Group Consortium PSMA • • • Type II transmembrane glycoprotein Expression on normal and neoplastic prostate epithelial cells, neovasculature Functions as a glutamate-preferring carboxypeptidase with two enzymatic activities: 1) Gamma-glutamyl carboxypeptidase (folate hydrolase 2) N-acetylated alpha-linked l-amino dipeptidase (NAALADase), an enzyme involved in regulation of excitation signaling Prostate Specific Membrane Antigen COOH COOH PSMA:extracellular form found on Prostate Specific Membrane Antigen cancer cells COOH E COOH PSMA:extracellular form found on cancer cells E COOH COOH F D E E D C C cell membrane B cell membrane B A A PSM’: intracellular form found in normal cells F Domain A - aa 1-19 Domain B – aa 20-39 E D C PSM’: intracellular form found in normal cells F Domain A - aa 1-19 Domain B – aa 20-39 COOH COOH F E D C RCC Bone (10mg) How to Target PSMA? Vaccines • Naked DNA • rsPSMA maytansinoid • Alphavirus vector Others: Radionuclide emitter + MoAb (ext domain) MoAb + Neovasculature? What is the ideal patient population? What is the appropriate clinical trial endpoint, i.e., does a clinical and/or immunologic endpoint exist? Metastatic population High titer Abs No effect on PSA Continued POD Rising PSA High titer Abs Change in PSA logslope Disease stabilization Rationale for targeting signalling cascades and surface receptors… a) Most prostate cancers have lost PTEN b) Tumors that have lost PTEN are insensitive to EGFr inhibition; Restoration of PTEN function (mTOR inhibition) restores sensitivity to EGFR inhibition c) PTEN negative tumors are sensitive to mTOR inhibition The combination of mTOR inhibition and EGFR inhibition may be a rationale treatment approach. Plasmid DNA expression vector used in MSKCC PSMA DNA vaccines kana cassette ori pCDNA3 polylinker pING+ HuPSMA CMV promoter 7213 bp CAAT TATA exon 1 PSMA ORF Human PSMA Intron A T7 Why HLA A02.01? • HLA-A02.01 allele: – Peptides that match the HLA-A2.01 binding consensus are found within the huPSMA and muPSMA – Expressed by 40% of the Caucasian population • To date: 126 patients typed: 65 + (52%) MSKCC Cross-over to break tolerance Cross-over design of clinical trial Human PSMA Human PSMA Mouse PSMA Mouse PSMA Why a DNA Vaccine? • Relatively inexpensive & simple to purify in large quantity • Avoids complex ex vivo expansion and manipulation of patients’ cells • Antigen of interest is cloned into a bacterial expression plasmid with a constitutively active promoter. • Bacterial plasmid DNA itself contains immunostimulatory sequences (CpG motifs) that may act as an immunological adjuvant • Direct entry of the antigen into the intracellular MHC class I processing pathway How to Break Immune Tolerance to PSMA? Active Passive Vaccines • Naked DNA Others: Radionuclide emitter + MoAb (ext domain) MoAb + maytansine Neovasculature • • • • rsPSMA Alphavirus vector Cytokine/GM-CSF (transduced cell line) ACP-fusion protein (cellular product) CD28/B7 superfamily and the paradigm of professional costimulation T cell APC/DC B7x BTLA YYY SHP-1 B7H3 SHP-2 Activation CD28 YYYY B7.1 Inhibition B7.2 CTLA-4 YY B7h ICOS YY PD-L1 PD-1 YY IgC domain Induced by Inflammation/ Pathogens PD-L2 IgV domain Y Tyrosine Regulation of T cell activation TCR Antigen MHC ~ CD28 CTLA-4 : B7 suppression Termination of response B7 TCR CD28 Antigen Antigen-specific T cell Activation MHC CTLA-4 ~ B7 TCR TCR : Antigen MHC CD28 : B7 Co-stimulation CD28 Antigen Activated T cell IL-2 secretion Proliferation Effector function Induction of CTLA-4 MHC ~ B7 CTLA-4 CTLA-4 Blockade Augments Antigen Specific T-cell Responses Anti-CTLA-4 mAb TCR CD28 Antigen MHC TCR CD28 ~ Antigen B7 MHC ~ B7 CTLA-4 CTLA-4 An activated T cell is important in destroying cancer cells or virally infected cells. CTLA-4 (in red) is sequestered in granules distal to the leading edge of the immunological synapse. Image courtesy of the Allison lab, University of California, Berkeley CTLA-4 A T-cell interacts with an antigen presenting cell (APC) and is activated. Preformed CTLA-4 granules (in red) migrate to the synapse. Signals downmodulate T-cell responses against cancers and other foreign antigens. Image courtesy of the Allison lab, University of California, Berkeley Anti-Murine CTLA-4 mAb Cures Prostate Cancer in Mice 300 Source: Kwon et al. PNAS. 1997(94): 8099 Control Ab Tumor size (mm 2) Anti-CTLA-4 (100 ug Ab at days 7, 10, & 13) 0 10 30 50 70 Days post tumor injection 90 α-CTLA-4 5000 4500 4000 CA-125 3500 3000 2500 α-CTLA-4 2000 1500 1000 500 0 -120 GVAX -100 -80 -60 -40 -20 0 20 40 60 80 Days 100 120 140 160 180 200 220 240 260 Pathology of Autoimmune Breakthrough Events: Dermatitis B A Histopathologic analyses of selected patients experiencing autoimmune events. (A) Skin rash biopsy from Patient 2 illustrating severe dermatitis with epidermal spongiosis, significant papillary dermal edema and a prominent inflammatory infiltrate in both the superficial and deep dermis (10X magnification). (B) The cellular infiltrate from Patient 2 reveals the predominance of lymphocytes, monocytes and eosinophils (40X). Source: Abstract #3424, ASCO 2003 Pathology of Autoimmune Breakthrough Events: Colitis D C E CD4 F CD3 CD8 Histopathologic analyses of selected patients experiencing autoimmune events. (C) Colon biopsy from Patient 9 illustrating severe colitis with infiltration of the lamina propria with neutrophils, lymphocytes, monocytes, plasmacytes and eosinophils. Neutrophils and lymphocytes also infiltrate the crypts; numerous mitotic figures can be seen in the epithelial cells lining the crypts (20X). Immunohistochemistry evaluating expression of CD3+ (D), CD4+ (E), and CD8+ markers (F) (20X). Source: Abstract #3424, ASCO 2003